Which compound in the following pairs will react faster in S2N reaction?
(a). CH3Br or CH3I
(b). (CH3)3CCl or CH3Cl
(a). CH3−I will react faster than CH3−Br because I− ion is a better leaving group than Br− ion. Alternatively, bond dissociation enthalpy of C-I bond is less than tthat of C-Br bond.
(b). CH3Cl will react faster because of less steric hindrance as compared to (CH3)3CCl.
Similar Questions
Which compound in each of the following paris will react faster in SN2 reaction with HOo− ?
a. CH_(3)BrorCH_(3)I<br>b.(CH_(3))_(3)CClorCH_(3)Cl<br>c.CH_(2) = CHBrorCH_(2) = CH - CH_(2)Br
View SolutionWhich compound in each of the following pairs will react faster in SN2 reaction with –OH ?
(i) CH3BrorCH3I,(ii)(CH3)3CClorCH3Cl
View Solution
Knowledge Check
Which compound in each of the following paris will react faster in SN2 reaction with HOo− ?
a. CH_(3)BrorCH_(3)I<br>b.(CH_(3))_(3)CClorCH_(3)Cl<br>c.CH_(2) = CHBrorCH_(2) = CH - CH_(2)BrA B
B C
C D
D
Which compound in each of the following pairs will react faster in SN2 reaction with -OH ? Why?
(i) CH3BrorCH3I (ii) (CH3)3CClorCH3Cl
View Solution(a) Draw the structures of major monohola prodcuts in each of the following reactions : 3
(i)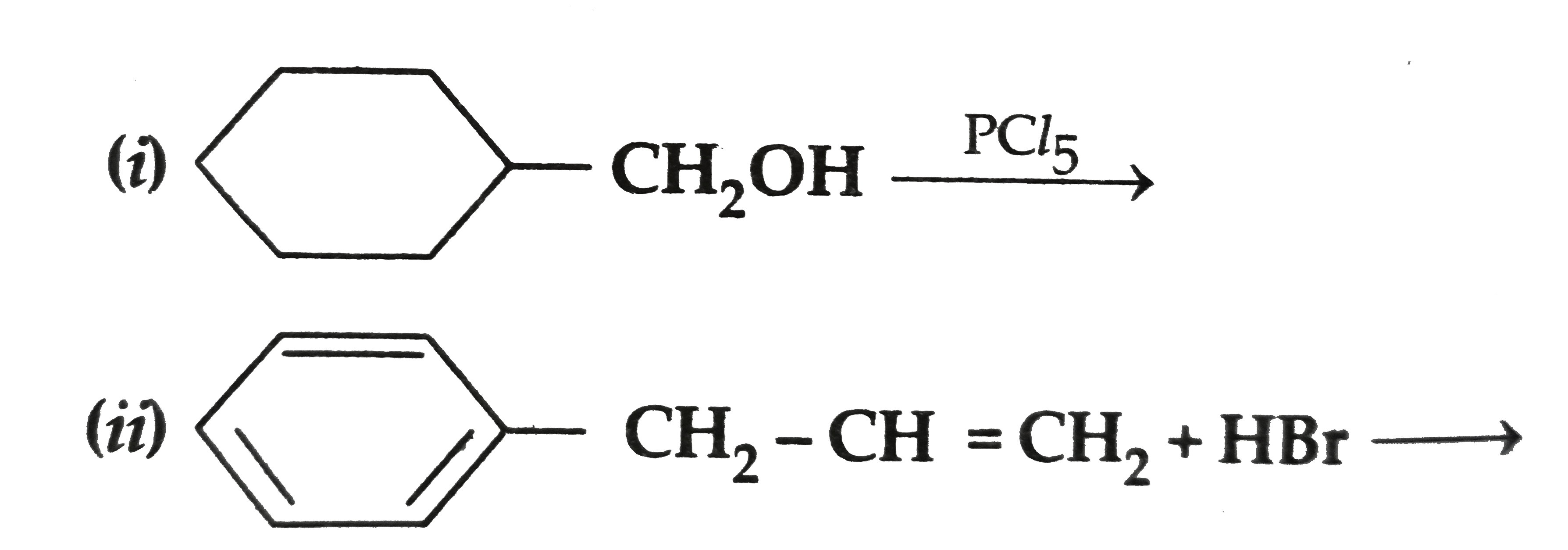
(b) Which halogen compound in each following pairs will react faster in SN2 reaction
(i) CH3Br or CH3I
(ii) CH3C−CI or CH3−CI
View SolutionWhich compound in each of the following pairs will react faster in SN2 reaction with –OH ?
(i) CH3BrorCH3I,(ii)(CH3)3CClorCH3Cl
View SolutionWhich halogen compound in each of the following pairs will react faster in SN2 reaction :
(i)CH3Br or CH3I (ii)(CH3)3C−Cl or CH3−Cl
View SolutionWhich compound in each of the following pairs will react faster in SN2 reaction with −OH :
(i) CH2Br or CH3I (ii) (CH3)3CCI or CH3CI ?
View SolutionWhich compound in each of the following pairs will react faster in SN2 reaction with OH−?
(a) CH3Br or CH3I
(b) CH2=CHBr or CH2=CHCH2Br.
View SolutionWhich compound in each of the following pairs will react faster in SN2 reaction with −OH ?
(i)CH3Br or CH3I (ii)(CH3)3CCl or CH3Cl
View Solution

