Complete the following:
CH3COCH3+I2+NaOH(warm)−−−−→.
To complete the reaction of acetone (CH₃COCH₃) with iodine (I₂) and sodium hydroxide (NaOH) upon warming, we follow these steps:
1. Identify the Reactants:
The reactants are acetone (CH₃COCH₃), iodine (I₂), and sodium hydroxide (NaOH). Acetone is a methyl ketone.
2. Recognize the Reaction Type:
The reaction involves the iodoform reaction, which occurs with methyl ketones in the presence of iodine and a base (NaOH).
3. Write the Reaction:
The methyl ketone (acetone) reacts with iodine in the presence of sodium hydroxide. The reaction can be summarized as:
CH3COCH3+3I2+4NaOH→CHI3+CH3COONa+3NaI+3H2O+CO2
4. Identify the Products:
- Iodoform (CHI₃)
- Sodium acetate (CH₃COONa)
- Sodium iodide (NaI)
- Carbon dioxide (CO₂)
- Water (H₂O)
5. Balance the Reaction:
Ensure that the number of atoms of each element is the same on both sides of the equation. The balanced equation is:
CH3COCH3+3I2+4NaOH→CHI3+CH3COONa+3NaI+3H2O+CO2
Final Answer:
The complete reaction is:
CH3COCH3+3I2+4NaOHwarm→CHI3+CH3COONa+3NaI+3H2O+CO2
The reactants are acetone (CH₃COCH₃), iodine (I₂), and sodium hydroxide (NaOH). Acetone is a methyl ketone.
The reaction involves the iodoform reaction, which occurs with methyl ketones in the presence of iodine and a base (NaOH).
The methyl ketone (acetone) reacts with iodine in the presence of sodium hydroxide. The reaction can be summarized as:
CH3COCH3+3I2+4NaOH→CHI3+CH3COONa+3NaI+3H2O+CO2
- Iodoform (CHI₃)
- Sodium acetate (CH₃COONa)
- Sodium iodide (NaI)
- Carbon dioxide (CO₂)
- Water (H₂O)
Ensure that the number of atoms of each element is the same on both sides of the equation. The balanced equation is:
CH3COCH3+3I2+4NaOH→CHI3+CH3COONa+3NaI+3H2O+CO2
The complete reaction is:
CH3COCH3+3I2+4NaOHwarm→CHI3+CH3COONa+3NaI+3H2O+CO2
Similar Questions
Knowledge Check
Complete the following reaction :
CH3COONa+NaOHCaO−−→ACH3COONa+NaOHCaO−−→NaOHBCH3COONa+NaOHCaO−−→CH3COOH+Ca(OH)2CCH3COONa+NaOHCaO−−→CH4+Na2CO3DCH3COONa+NaOHCaO−−→CH3COOCa+NaOHWhat is the major product obtained from the following reactions ?
CH3COCH2COOHheat−−→ productACH3CHO+CH3COOHBCH3COCH3CCH3−OH∣C=CH2D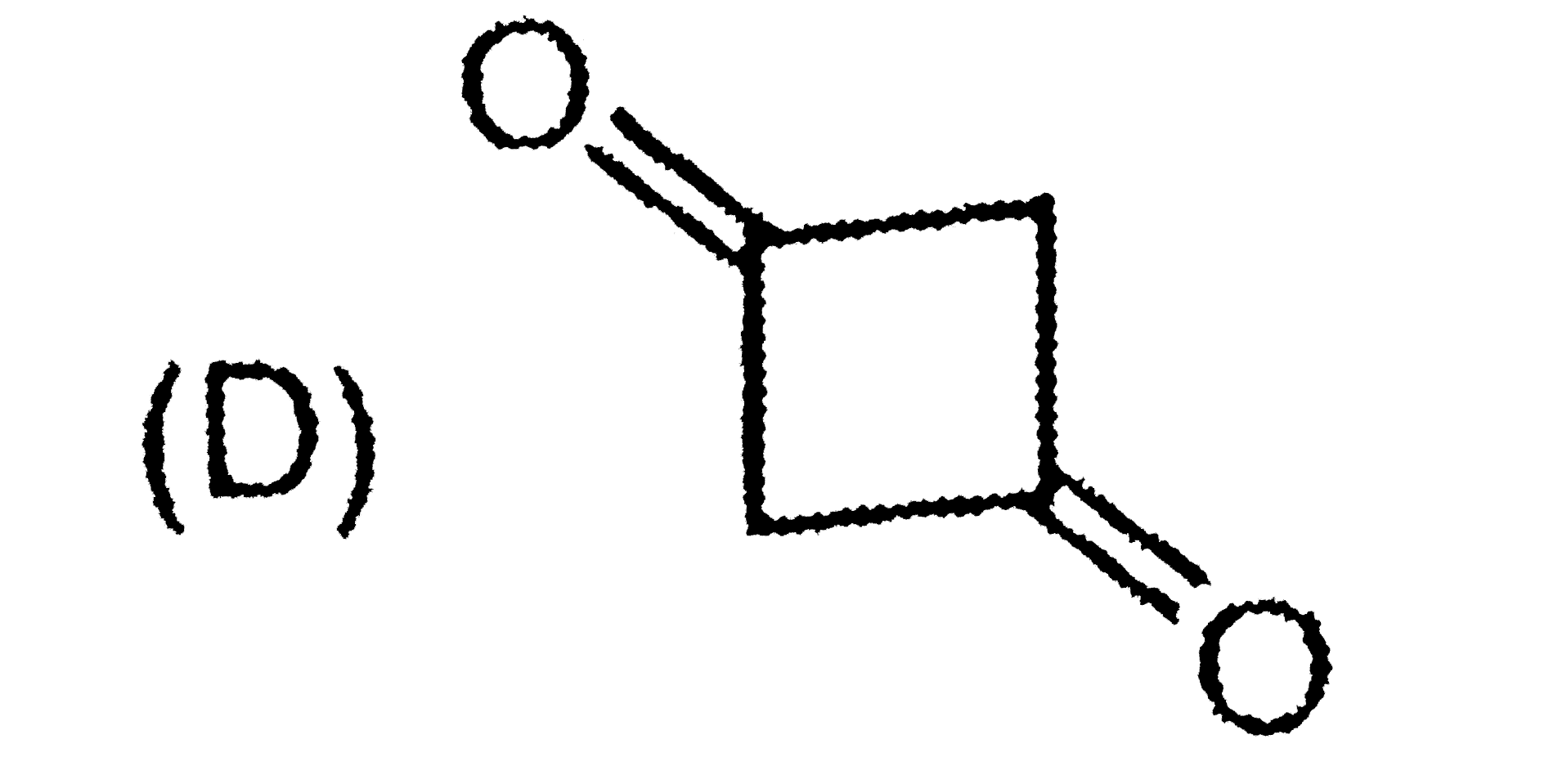
Complete the following :
(ii) CH3CONH2+NaOH+Br2→
View SolutionComplete the following reactions:
(i) CH3−CH2−O−CH3| C | CH3−CH3−HIHeat−−→
View SolutionComplete the reaction :
CH3COONa+NaOHCaO−−→
View SolutionComplete the following sequence of reactions :
CH3COCH3Ba(OH)2⇐====⇒(A)Δ−−−−−→−H2O(B)NaOH,I2−−−−−→(C)+(D)
(i) Identify (A) to (D).
(ii) Give the IUPAC name of (A).
View SolutionComplete the following
2HCHO+NaOH→
CH3CHO+H3Pd/BaSO4−−−−−−→
View SolutionComplete the following chemical equations :
(i) CH3COOC2H5+NaOH→
(ii) CH3COOH+NaOH→
(iii) C2H5OH+CH3COOHConc. H2SO4−−−−−−−−→
View Solution
