Predict titration curve for titration of Na(2)CO3 & HCl
Text Solution
Na2CO3+HCl→NaHCO3+NaCl
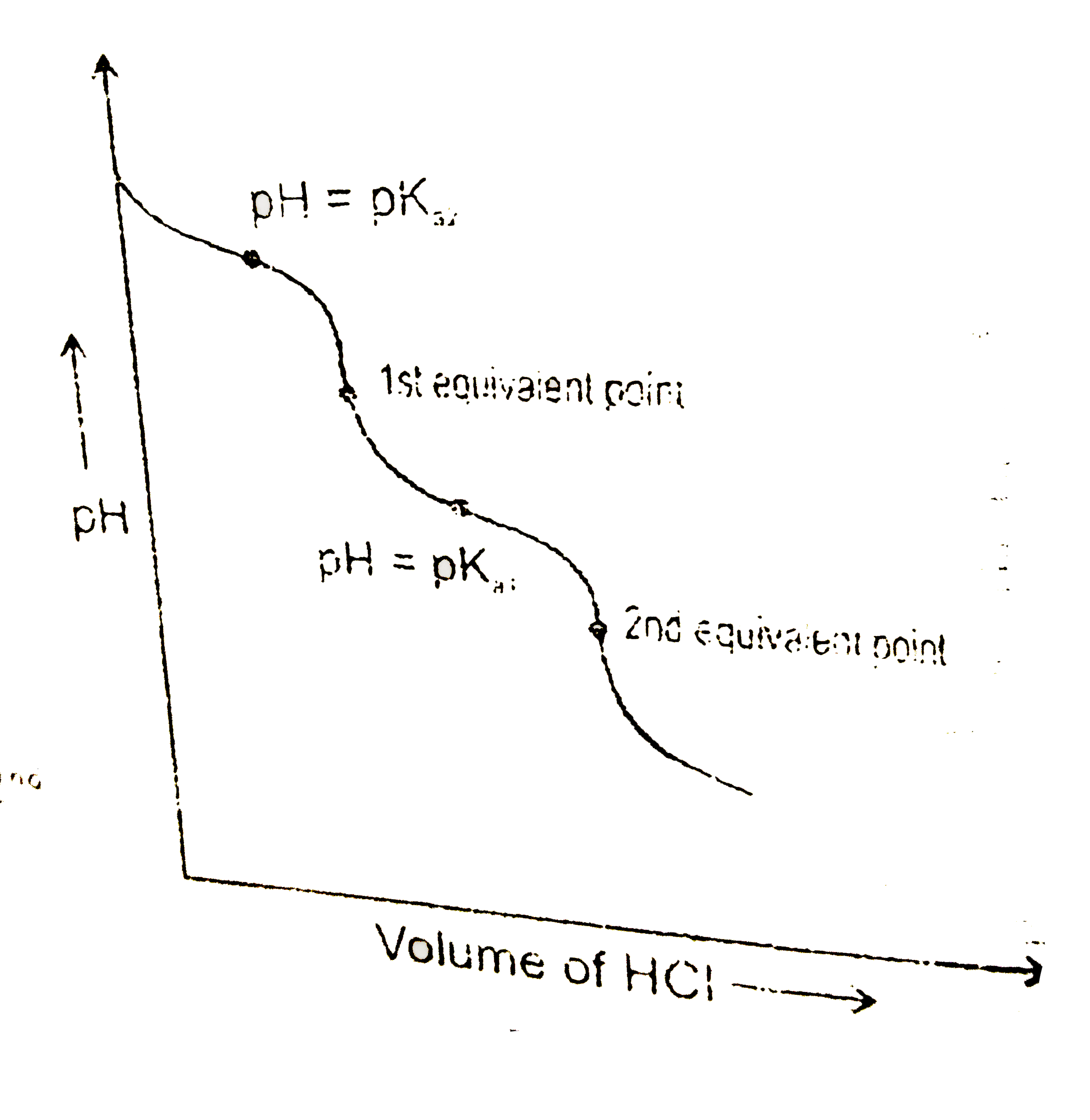
pH1=Half equivalence point with respect to1st neutralisation =pKa2(:.solution will be a buffer consisting of equal amount of CO2−3&HCO−3)
pH1steqpt=pKa1+pKa22(∴amphiprotic anion)
NaHCO3+NaCl→H2CO3+NaCl
pH2=half equivalence point with respect to 2ndneutralisation =pKa2 ( :.solution will be a buffer)
consisting of equal amount of HCO−3&H2CO3)
pH2ndaqpt=1/2(pKa1−logC)(:.weak acid)
|
Similar Questions
Knowledge Check
If we use phenolphthalein as an indicator in a titration of Na2CO3 with HCl, the usual result is
ANo visible change is occurBThe indicator reacts with the acidCThe indicator reacts with the baseDSodium chloride and carbonic acid will be formedDuring the titration of a mixture of Na2CO3 and NaHCO3 against HCl :
APhenolphthalein is used to detect the first end pointBPhenolphthalein is used to detect the second end pointCMethyl orange is used to detect the first end pointDPhenolphthalein is used to detect the first and second end pointAssertion: In the titration of Na2CO3 with HCl using methyl orange indicator, the volume of acid required is twice that of the acid required using phenolphthalein as indicaton.
Reason: Two moles of HCl are required for the complete neutralisation of one mole of Na2CO3.A(a)If both assertion and reason are true and the reason is the correct explanation of the assertion.B(b)If both assertion and reason are true and the reason is not the correct explanation of the assertion.C(c )If assertion is true but reason is false.D(d)If assertion is false but reason is true.
A solution contains Na2CO3 and NaHCO3 . 20 ml of this solution required 4 ml of 1 N HCl for titration with Ph indicator. The titration was repeated with the same volume of the solution but with MeOH. 10.5 ml of 1 N HCl was required this time. Calculate the amount of Na2CO3 & NaHCO3.
View Solutiontitration(introduction),redox titration
View Solutionconductometric titration curve of a equimolar mixture of a HCl and HCN with NaOH(aq) is :
View SolutionWhich of the following indicators is used in the titration of Na2S2O3 against CuSO4 solution?
View SolutionThe best indicator for titrating 0.1 Na2CO3 against 0.1 N HCl is
View Solution

