Silicone resins are made by:
A
dissolving a mixture of PhSiCl3 and (Ph)2SiCl2 in toluene and then hydrolysis with water.
B
hydolysing a mixture of (CH3)2SiCl2 and (CH3)3SiCl
C
hydrolysis of (CH3)2SiCl2
D
none
Video Solution
Text Solution
The correct Answer is:A
(A)When a mixture of PhSiCl3 and (Ph)2SiCl2 is dissolved in toluene and then hydrolysed with water silicon resins are obtained.
(B)Silicones from the hydrolysis of a mixture of (CH3)3SiCl & (CH3)2SiCl2
The dichloro derivative will form a long chain polymer as usual.But the growth of this polymer can be blocked at any stage by the hydrolysis product of mono-chloro derivative.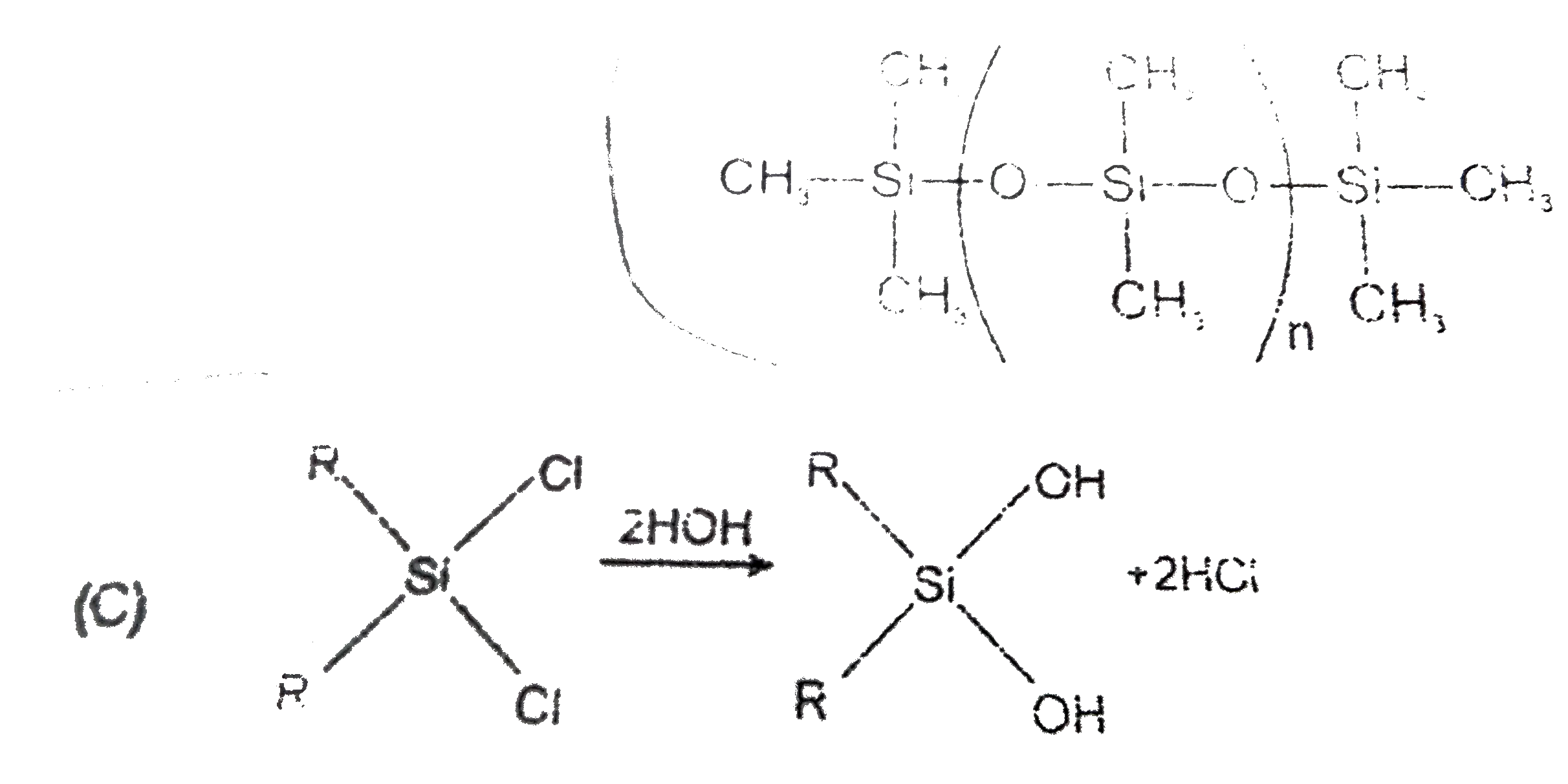
OH−R∣Si∣R−OH+OH−R∣Si∣R−OH−−−→−H2OOH−R∣Si∣R−O−R∣Si∣R−OH
In this manner several molecules may combine to form a long chain polymer whose both the ends will be occupied by −OH groups.Such compounds are generally represented from the following formula.
OH−R∣Si∣R−O−R∣Si∣R−O−R∣Si∣R−O−
|
Similar Questions
Knowledge Check
Silicones are
AToxicBNon-ToxicCBitterDSourSilicones are
ASynthetic polymers containing repeated R2SiO unitsBSilicates with common SiO4 unitCKetones with silyl group (SiH3) similar to alkyl, (SiH3)3CODZircon (meso Silicates )Silicon is
Asemi-conductorBconductorCInsulatorDNone of these
