The correct statement (s) for orthoboric acid is/are
A
It behaves as a weak acid in water due to self ionization.
B
Acidity of its aqueous solution increases upon addition of ethylene glycol.
C
It has a three dimensional structure due to hydrogen bonding.
D
It is weak electrolyte in water.
Video Solution
Text Solution
The correct Answer is:B, D
H3BO3 does not undergo self ionization.
On adding cis-diols, them form complexing species with orthoboric acid.
Hence the acidity increase on adding ethylene glycol.
H3BO3+H2O→B(OH)−4+H+
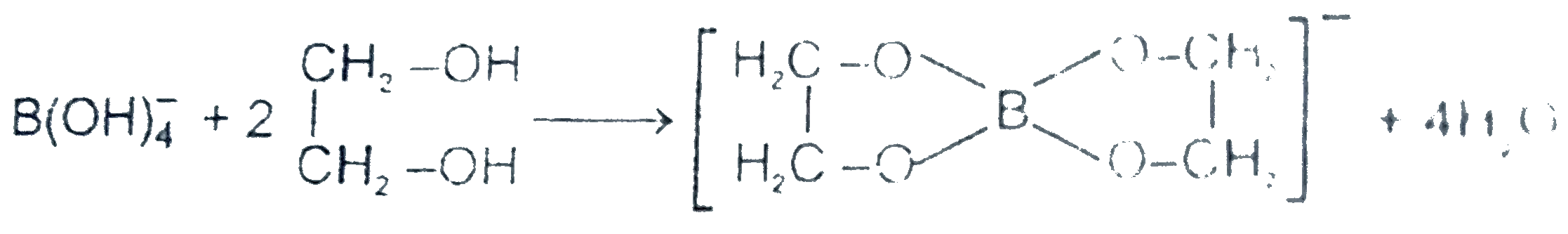
It arranges into planar sheets due to H-bonding.
Hence, it has 2-dimensional structure due to H bonding.
It acts as a weak acid in water, so it is a weak electrolyte in water
|
Similar Questions
Knowledge Check
The correct statement about orthoboric acid is
AIt is a strong monobasic acidBit is not a proton donor, but a weak Lewis acidCIt is a tribasic acidDIt is harmful for eyesThe correct statement (s) is/are:
AAll system maximze their entropy at equilibriumBAl substance have zero entropy at absolute zero temperatureCAt constant P,T if ΔG<0 then the process must be work producingDIn all adiabatic processes, entropy of surroundings is constant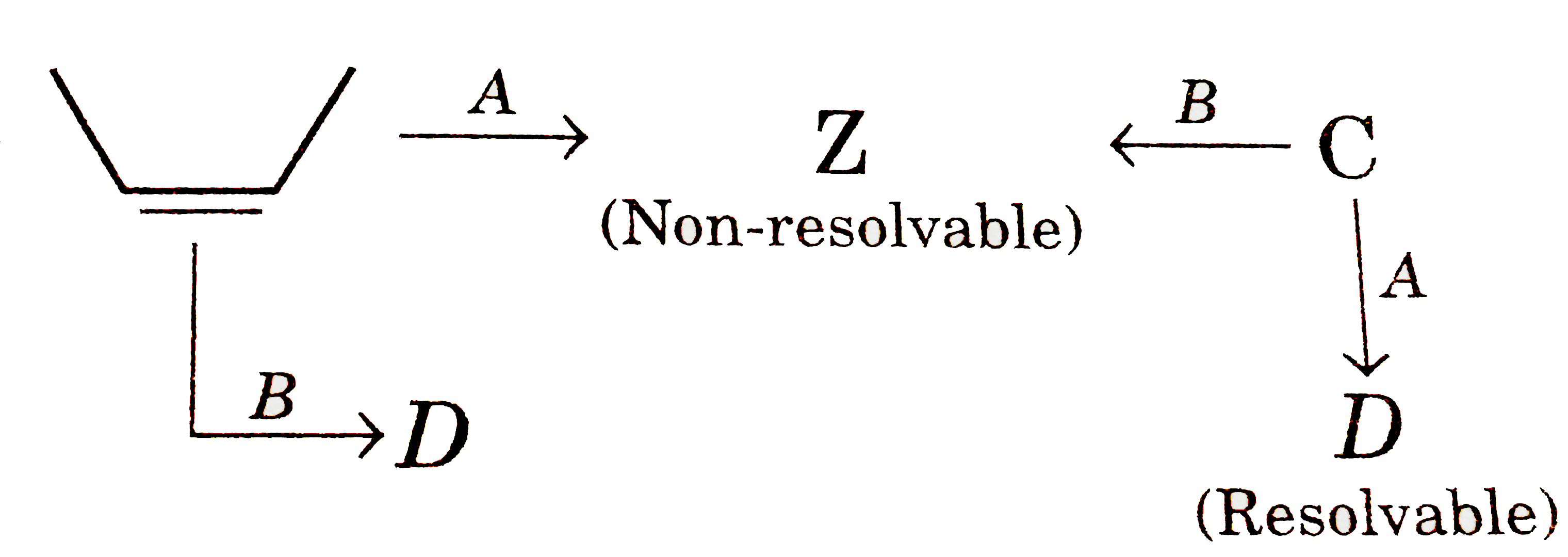
Select correct statement (s) :AA is OsO4/aq.NaHCO3.BB is mCPBA/H3O⊕.CC is obtained when D is heated with conc. H2SO4.DReaction C to Z is stereo non-selective.

