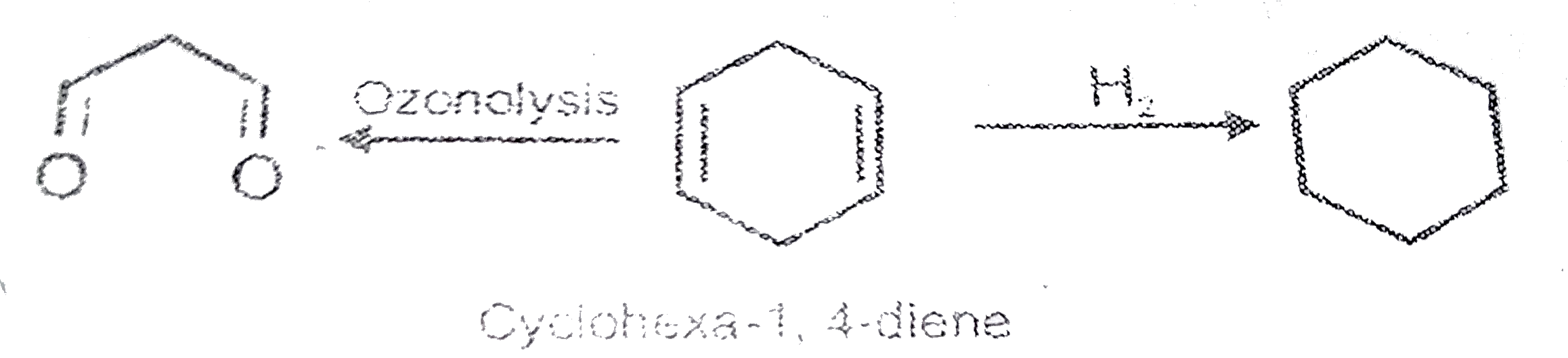
A hydrocarbon of formula C6H8 absorbs two mole of H2 upon hydrogenation. On ozonolysis, it yields Propanedial. What is hydrocarbon ?
Hydrocarbon is Cyclohexa-1,4-diene.
Similar Questions
A hydrocarbon of formula C10H16 absorbs only one mole of H2 upon hydrogenation. On ozonolysis, it yields 1, 6-Cyclodecanedione. What is the structure of the hydrocarbon ?
View SolutionA hydrocarbon of formula C10H16 absorbs only one mole of H2 upon hydrogenation. On ozonolysis, it yields 1, 6-Cyclodecanedione. What is the structure of the hydrocarbon ?
View Solution
Knowledge Check
A hydrocarbon of formula C6H10, absorbs only one molecule of H2 upon catalytic hydrogenation. Upon ozonolysis the hydrocarbons yeilds.
OHC−CH2−CH2−CH2−CHO the compound is:AcyclohexeneBcyclohexaneCbenzeneDcyclobutaneA hydrocarbon of formula C6H10 absorbs only one molecule of H2 upon catalytic hydrogenation. Upon ozonolysis the hydrocarbon yields
O=H∣C−CH2−CH2−CH2−CH2−H∣C=O
The hydrocarbon isAcyclohexaneBbenzeneCcyclohexeneDcyclobutaneA hydrocarbon of formula C6H10, absorbs only one molecule of H2 upon catalytic hydrogenation. Upon ozonolysis the hydrocarbons yeilds.
OHC−CH2−CH2−CH2−CH2−CHO the compound is:ACyclohexaneBBenzeneCCyclohexeneDCyclobutane
One mole of a compound with molecular formula C30H43N absorbs 8 moles of H2 gas under catalytic hydrogenation. Then what is the ratio of number of π bonds to the number of rings in the compound
View SolutionOne mole of a compound with molecular formula C30H43N absorbs 8 moles of H2 gas under catalytic hydrogenation. Then what is the ratio of number of π bonds to the number of rings in the compound
View SolutionA hydrocarbon with formula C8H18 gives one monochloro derivative. The hydrocarbon can be:
View SolutionA hydrocarbon with formula C8H18 gives one monochloro derivative. The hydrocarbon can be:
View SolutionA hydrocarbon with formula C8H12 gives one monochloro derivative , the hydrocarbon is:
View Solution

