When one mole of the given compond reacts with sodium metal then how many moles of H2 gas will release ?
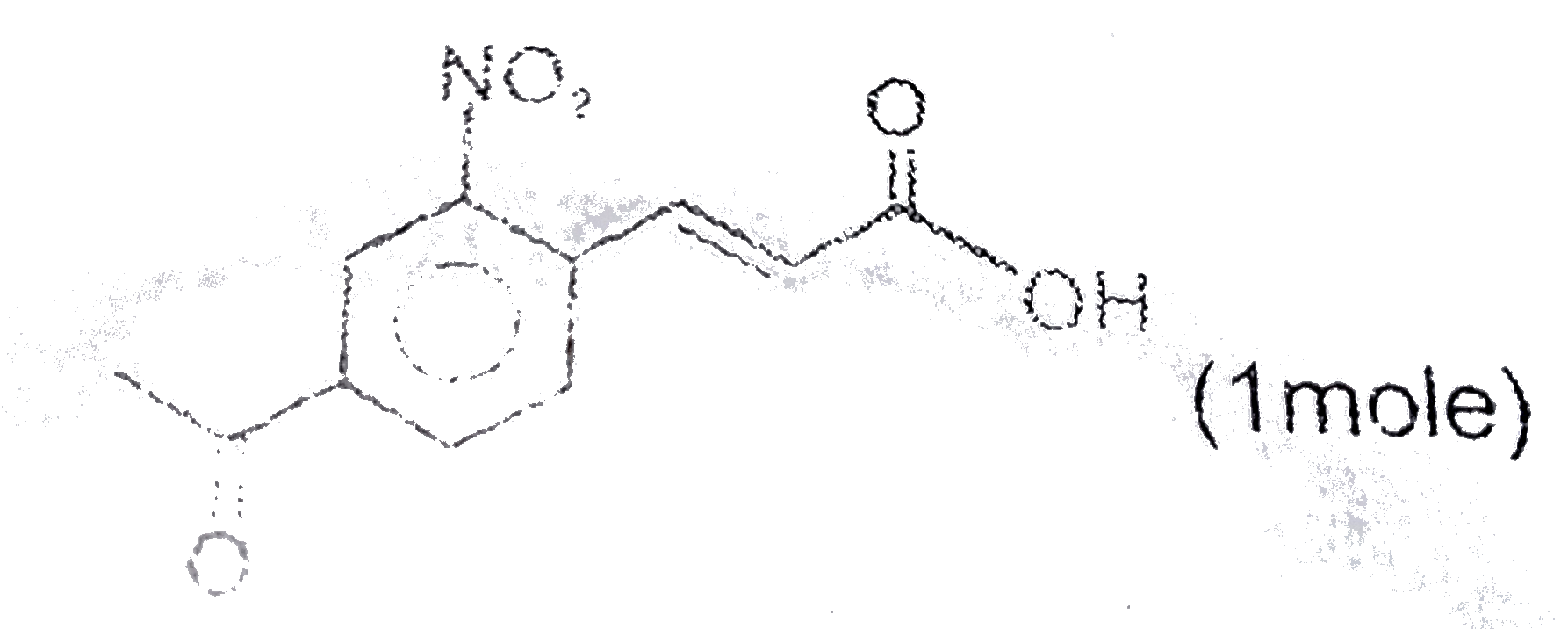
A
1 mole
B
1.5 mole
C
2 mole
D
0.5 mole
Text Solution
The correct Answer is:D
|
Similar Questions
When C_(2)H_(5)OH reacts with sodium metal, gas released is
View SolutionWhen white phosphorous react with NaOH a gas is produced which is pass through AgNO3 solution.Then how many mole of AgNO3 react with per mole of gas?
View Solutionनिम्न में से कौनसा यौगिक सोडियम धातु के साथ अभिक्रिया के पश्चात् H2 गैस मुक्त करेगा ?
View Solutionनिम्न में से कौनसा यौगिक सोडियम धातु के साथ अभिक्रिया के पश्चात् H2 गैस मुक्त नहीं करेगा ?
View Solutionनिम्न में से कौनसा यौगिक सोडियम धातु के साथ अभिक्रिया के पश्चात् H2 गैस मुक्त नहीं करेगा ?
View SolutionHow many moles of H2O are liberated when one mole hydrated MgCl2 is heated?
View SolutionHow many moles of H2O are liberated when one mole hydrated MgCl2 is heated?
View SolutionNo. of moles of H_(2) gas evolved when one mole of the following compound reacts with sodium.
View Solution

