How many no. of active Hydrogen atoms are present in compound (mol. Mass 90) 0.45g of which when treated with Na metal liberates 112ml of the H2 gas at STP.
Text Solution
The correct Answer is:No. of active H=2
|
Topper's Solved these Questions
- STRUCTURAL IDENTIFICATIONBOOK - RESONANCECHAPTER - STRUCTURAL IDENTIFICATIONEXERCISE - Exercise - 2 (Part-III)
6 Videos
- STRUCTURAL IDENTIFICATIONBOOK - RESONANCECHAPTER - STRUCTURAL IDENTIFICATIONEXERCISE - Exercise - 2 (Part-IV)
3 Videos
- STRUCTURAL IDENTIFICATIONBOOK - RESONANCECHAPTER - STRUCTURAL IDENTIFICATIONEXERCISE - Exercise - 2 (Part-I)
11 Videos
- STEREOISOMERISMBOOK - RESONANCECHAPTER - STEREOISOMERISMEXERCISE - EXERCISE (PART III : PRACTICE TEST-2 (IIT-JEE (ADVANCED PATTERN))
22 Videos
- SURFACE CHEMISTRYBOOK - RESONANCECHAPTER - SURFACE CHEMISTRYEXERCISE - Section - 5
1 Video
Similar Questions
Knowledge Check
How many 2∘ Hydrogen atoms are present in the given following compound ?
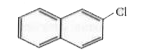 A2B5C7D8
A2B5C7D8How many 2∘ Hydrogen atoms are present in the given following compound ?
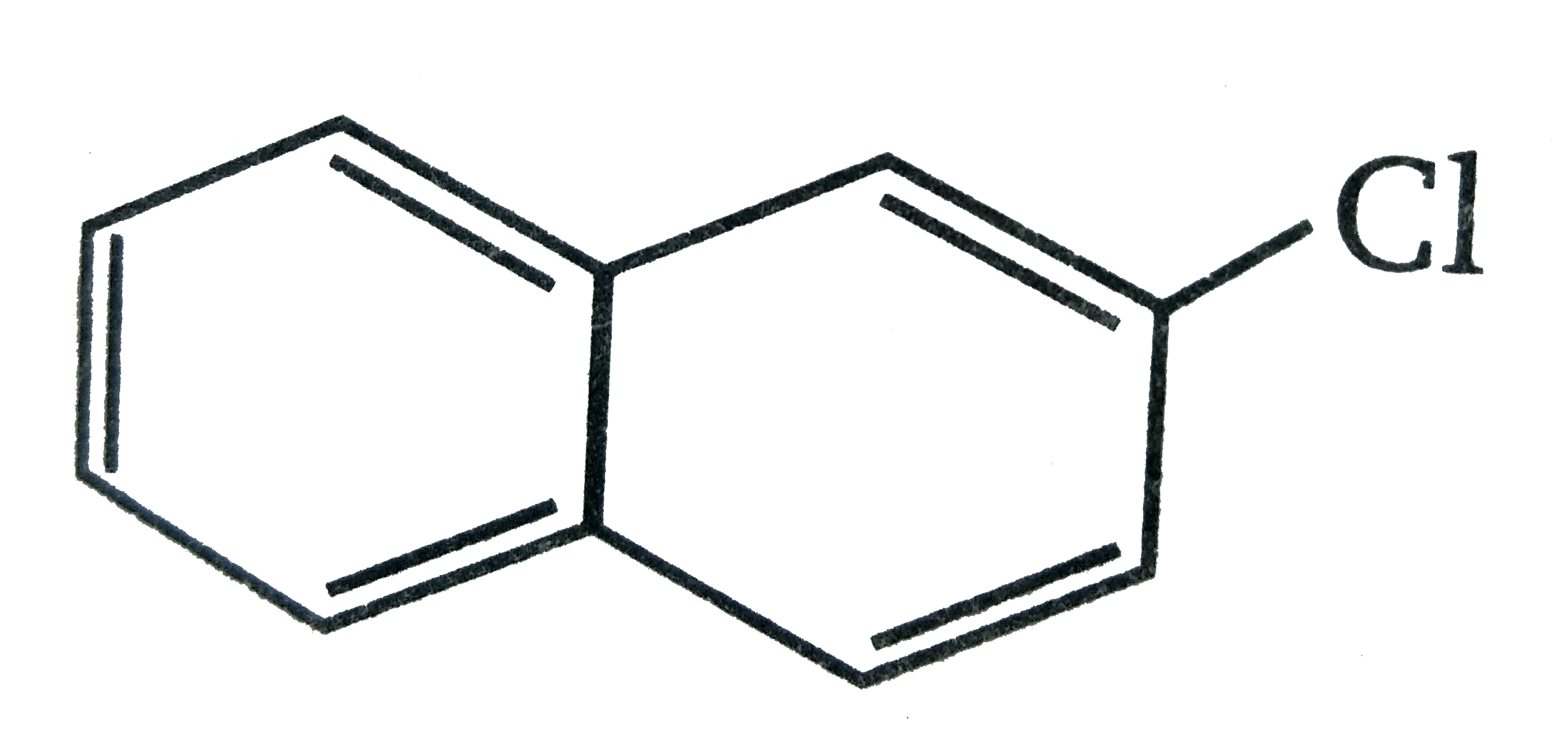 A2B5C7D8
A2B5C7D8How many H− atoms are present in 0.046g of ethanol ?
A6×1020B1.2×1021C3×1021D3.6×1021
How many molecules of SO2 are present in 11.2 L at STP?
View SolutionHow many H− atoms are present in 0.046g of ethanol ?
View SolutionWhich of the following compound is/are react with Na metal & liberate hydrogen gas
View SolutionWhen 1 mol of ethanol reacts with sodium metal liberate how many gram of hydrogen ?
View SolutionThe compound which liberates H2 gas with sodium metal is ,
View Solution

