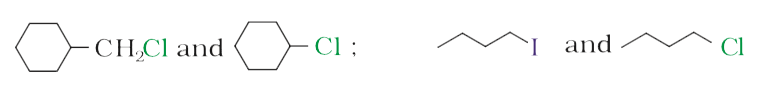Predict the compound which will undergo SN2 reaction faster:
CH3−CH2−CH2−Cl
H3C−Cl∣C−CH3
CH3−CH2−CH2−l
H3C−l∣CH−CH3
The correct Answer is:C
To determine which compound will undergo an SN2 reaction faster, we need to consider two main factors: the degree of the alkyl halide and the quality of the leaving group.
1. Identify the Types of Alkyl Halides:
- Alkyl halides can be classified as primary (1°), secondary (2°), or tertiary (3°). The reactivity in SN2 reactions follows the order: 1° > 2° > 3°.
- In this case, we have two compounds to compare:
- Option 1: CH3CH2CH2Cl (1° alkyl halide)
- Option 3: CH3CH2CH2I (1° alkyl halide)
2. Evaluate the Leaving Groups:
- The leaving group is crucial in determining the rate of the SN2 reaction. A better leaving group will facilitate a faster reaction.
- In this case, we have:
- Chlorine (Cl) in Option 1
- Iodine (I) in Option 3
- Iodine is a better leaving group than chlorine because it is larger and can stabilize the negative charge better once it leaves.
3. Combine the Factors:
- Both compounds are primary alkyl halides, which means they have the same steric hindrance and will react at similar rates based on their structure.
- However, since Option 3 has iodine as the leaving group, it will react faster than Option 1, which has chlorine.
4. Conclusion:
- Therefore, the compound that will undergo the SN2 reaction faster is Option 3: CH3CH2CH2I.
- Alkyl halides can be classified as primary (1°), secondary (2°), or tertiary (3°). The reactivity in SN2 reactions follows the order: 1° > 2° > 3°.
- In this case, we have two compounds to compare:
- Option 1: CH3CH2CH2Cl (1° alkyl halide)
- Option 3: CH3CH2CH2I (1° alkyl halide)
- The leaving group is crucial in determining the rate of the SN2 reaction. A better leaving group will facilitate a faster reaction.
- In this case, we have:
- Chlorine (Cl) in Option 1
- Iodine (I) in Option 3
- Iodine is a better leaving group than chlorine because it is larger and can stabilize the negative charge better once it leaves.
- Both compounds are primary alkyl halides, which means they have the same steric hindrance and will react at similar rates based on their structure.
- However, since Option 3 has iodine as the leaving group, it will react faster than Option 1, which has chlorine.
- Therefore, the compound that will undergo the SN2 reaction faster is Option 3: CH3CH2CH2I.
Similar Questions
Knowledge Check
SN1 reaction is faster in
ACH3CH2ClB CCH3−CH3∣C∣CH3−ClDCH3−CH∣∣CH2∣CH3−Cl
CCH3−CH3∣C∣CH3−ClDCH3−CH∣∣CH2∣CH3−ClThe compound which undergoes SN1 reaction most rapidly is :
A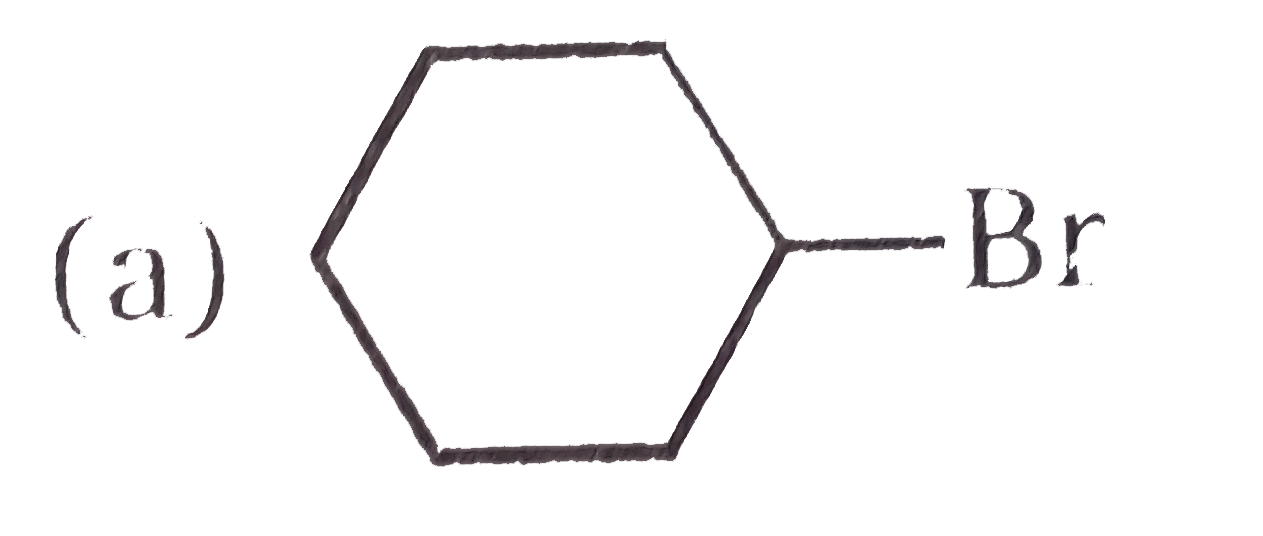 B
B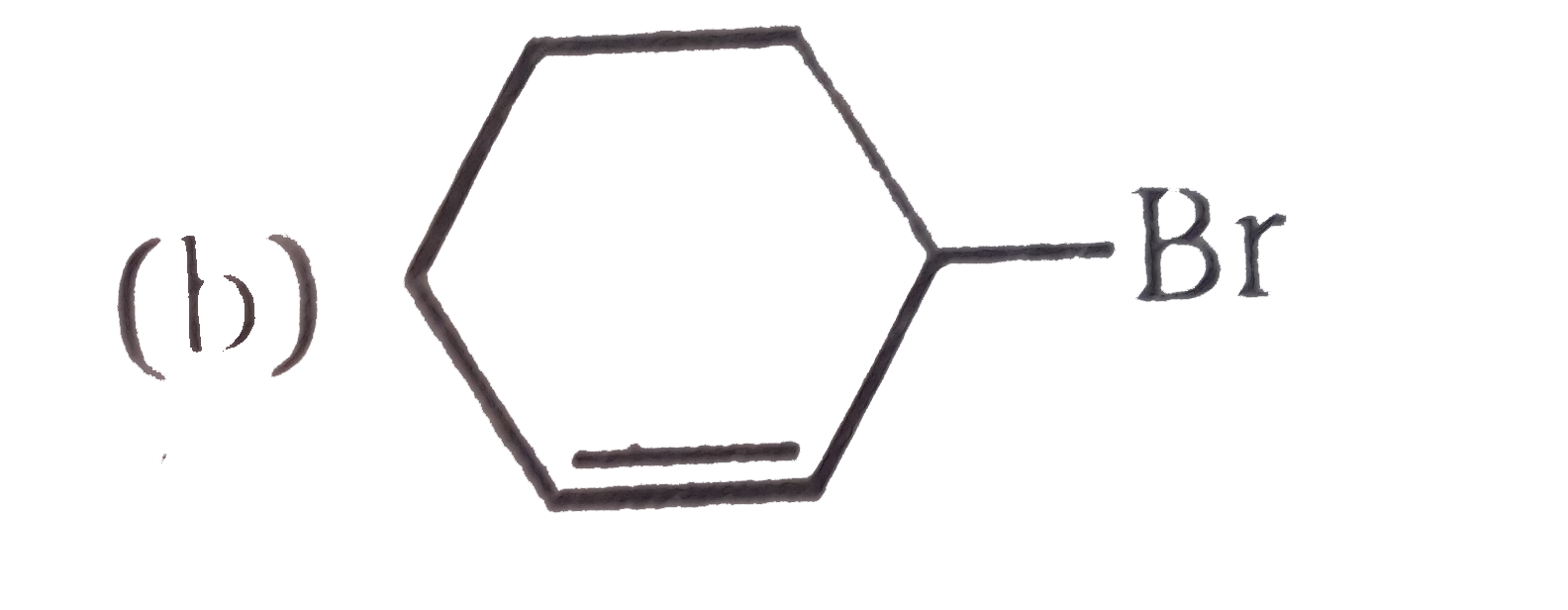 C
C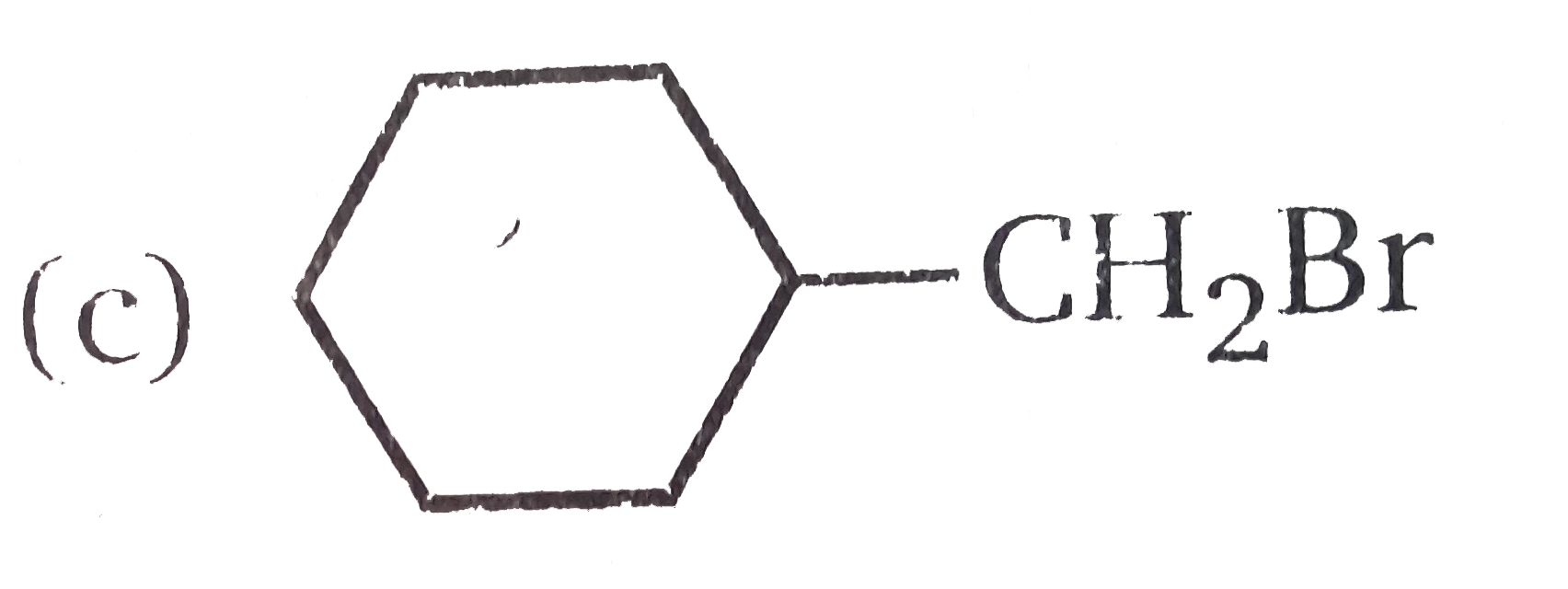 D
D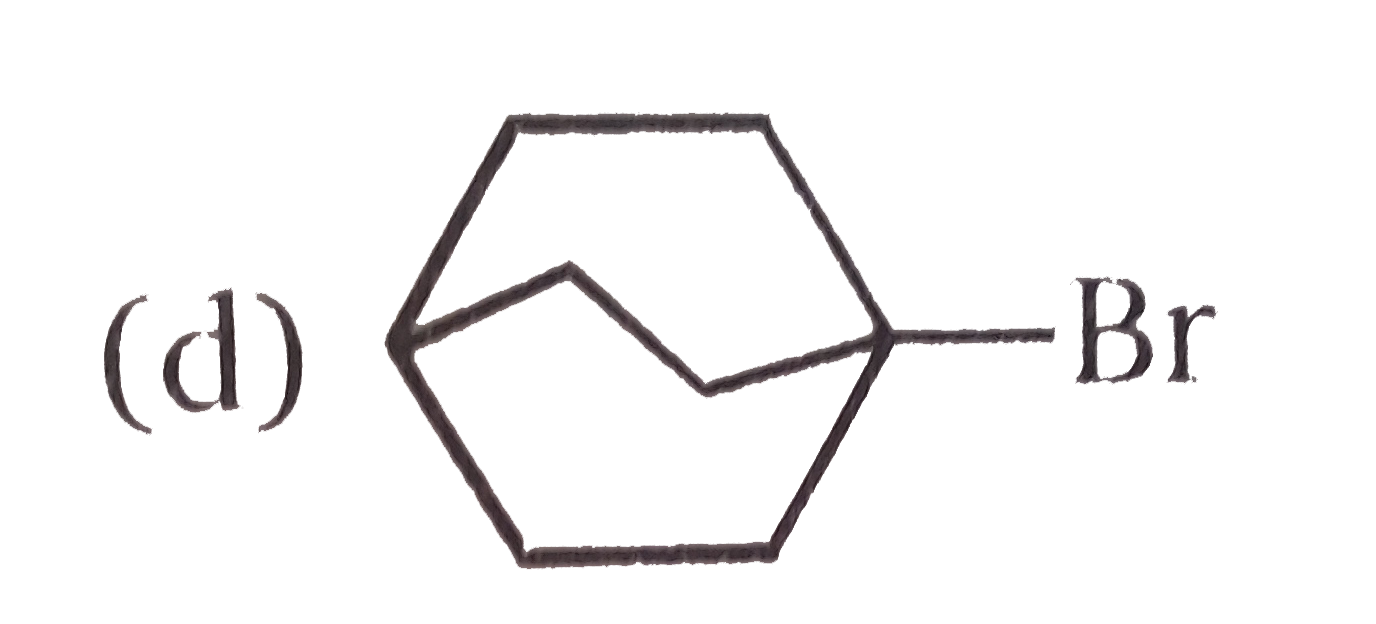
Predict the compound which will undergo solvolysis (aqueous ethanol) more rapidly.
ACH3−(CH2)2−CH−ClBH3C−Cl∣C∣CH3−CH3CH5C6−Cl∣C∣CH3−CH3DH3C−CH2−Cl∣CH−CH3

which of the given compounds would undergo SN1 reaction faster and why?
View SolutionIn the following pairs of halogen compounds, which compound undergoes SN1 reaction faster?

View SolutionWhich of the following compounds would undergo SN1 reaction faster and why ?
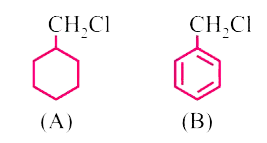
View SolutionWhich of the following compounds would undergo SN1 reaction faster and why ?
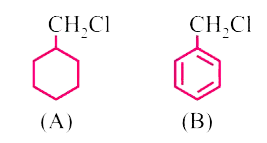
View SolutionAmong the following
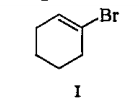
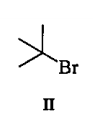
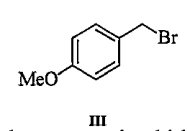
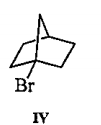 the compounds which can undergo an SN1 reaction in an aqueous solution, are
the compounds which can undergo an SN1 reaction in an aqueous solution, are
View Solution


 .
.