MnO−4+C2O2−4+H+→Mn2++CO2+H2O उपरोक्त उभयधर्मी अभिक्रिया (redox reaction) की संतुलित अभिक्रिया के लिए अभिकारकों के सही गुणांक हैं
A
MnO−4C2O2−4H+2516
B
MnO−4C2O2−4H+1652
C
MnO−4C2O2−4H+5162
D
MnO−4C2O2−4H+2165
Video Solution
Text Solution
The correct Answer is:A
2MnO−4+5C2O2−4+16H+→2Mn2++10CO2+8H2O
|
Similar Questions
What is the coefficient of oxalate ion in the following reaction ? MnO_(4)^(-)+C_(2)O_(4)^(-)+H^(+) to Mn^(2+)+CO_(2)H_(2)O
View SolutionFor the redox reaction,
MnO−4+C2O2−4+H+→Mn2++CO2+H2O
the correct coefficients of the reactants for the balanced reaction are
View SolutionIn the reaction C_(2) O_(4)^(-2) + MnO_(4)^(-) + H^(+) rarr Mn^(+2) +CO_(2) the reductants is -
View Solutionअभिक्रिया
MnO−4+C2O2−4+H+→Mn2++CO2+H2O
के लिये कौन-सा सही है ?
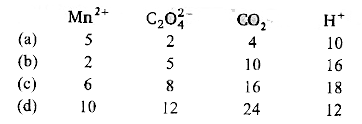
View SolutionIn the reaction : C_(2)O_(4)^(2-)+MnO_(4)^(-)+H^(+) to Mn^(2+)+CO_(2)+H_(2)O , the oxidising agent is
View SolutionCoefficients of MnO_(4)^(-), C_(2)O_(4)^(2-) and H^(+) in the balanced reaction, MnO_(4)^(-)+C_(2)O_(4)^(2-)+H^(+) to Mn^(2+)+CO_(2)+H_(2)O
View SolutionMnO−4+C2O2−4+H+=CO2+Mn2++H2O
View Solution

