यदि रैखिक समीकरण निकाय
x−4y+7z=g
3y−5x=h
−2x+5y−9z=k
संगत (consistent) हैं , तो
g+h+2k=0
g+h+k=0
g+2h+k=0
2g+h+k=0
The correct Answer is:D
Step by step video, text & image solution for यदि रैखिक समीकरण निकाय x-4y + 7z = g 3y -5x = h -2x + 5y -9z = k संगत (consistent) हैं , तो by Maths experts to help you in doubts & scoring excellent marks in Class 12 exams.
Updated on:21/07/2023Similar Questions
If the bond energies of H-H, Br-Br and HBr are 433,192and364kJmol−1 respectively, then ΔH∘ for the reaction :
H2(g)+Br2(g)→2HBr(g) is
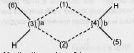
View SolutionWite down the corresponding elements in the figure : 1)……………… br 2)…………… br 3).……………. Br 4)………….. Br 5)…………. Br 6)…………..
View SolutionCalculate DeltaH_r^theta for reaction H_2(g)+Br_2(g)rarr2HBr(g) given that the bond energies of H-H, Br-Br and H-Br are 435, 192 and 364 kJ mol^-1 respectively.
View SolutionThe bond enthalpis of H-H, Br-Br and H-Br are 433, 192 and 364kJ/mol respectively. Calculate the enthalpy of the reaction H2(g)+Br2(g)→2HBr(g
View SolutionBr(g)+e−→Br− के लिए ΔH का मान होगा
View SolutionBr(g)+e−1→Br− के लिए ΔH का मान होगा
View Solutionअभिक्रिया H2(g)+Br2(g)→2HBr(g) के लिए ΔH∘ के मान ज्ञात करे यदि H - H, Br - Br तथा H-Br क्रमशः 433, 192 तथा 364 kJ मोल −1 हो।
View Solutionयदि H - H, Br - Br और H - Br की बंध ऊर्जा क्रमशः 433, 192 और 364 kJ mol−1 है, तो अभिक्रिया H2(g)+Br2(g)→2HBr(g) के लिए ΔH∘ का मान होगा :
View Solution

