Units of parts per million (ppm) or per billion (ppb) are often used to describe the concentrations of solutes in very dilute solutions. The units are defined as the number of grams of solution per million or per billion gram of solvent. Bay of Bengal has 2.1 ppm of lithium ions. What is the molality of Li+ in this water ? (Li = 7)
The correct Answer is:3.0×10−4
Step by step video, text & image solution for Units of parts per million (ppm) or per billion (ppb) are often used to describe the concentrations of solutes in very dilute solutions. The units are defined as the number of grams of solution per million or per billion gram of solvent. Bay of Bengal has 2.1 ppm of lithium ions. What is the molality of Li^(+) in this water ? (Li = 7) by Chemistry experts to help you in doubts & scoring excellent marks in Class 12 exams.
Updated on:21/07/2023Topper's Solved these Questions
Similar Questions
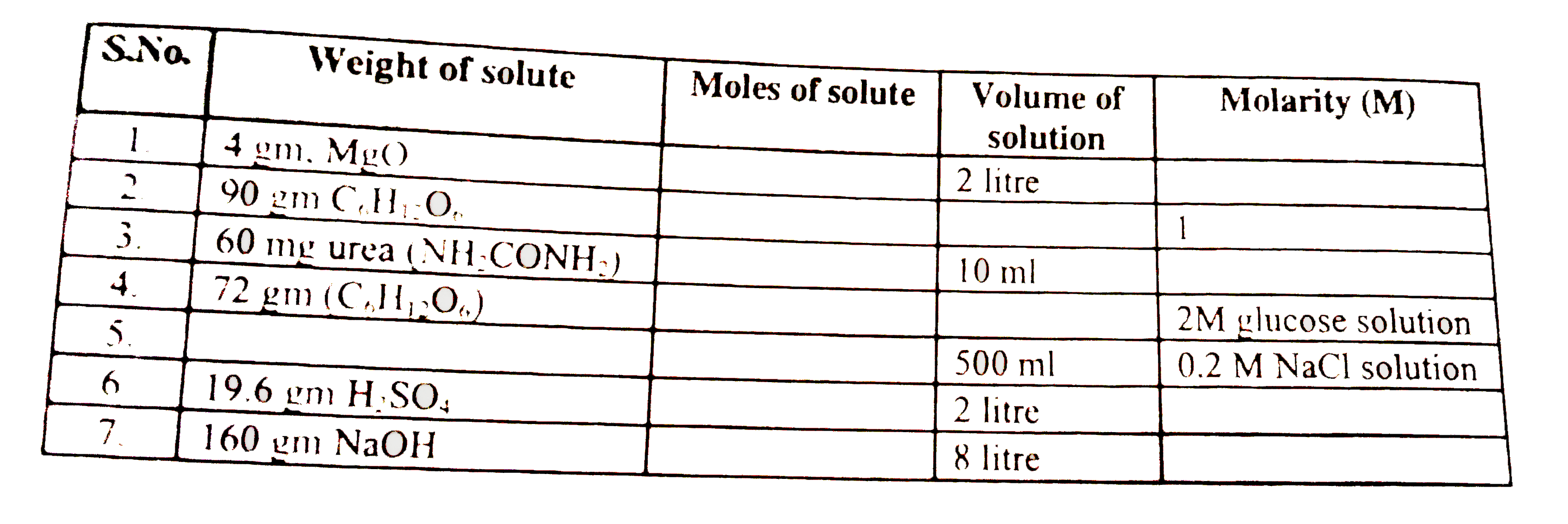
Complete the following table (all are aqueous solution)
View SolutionParts Per Million(PPM) || Parts Per Billion (PPB) ||Solved Numerical Question || Conversion OF 1 Unit into Another || Meaning OF Molal/Semi-molal/Deci-molal/Centi-molal
View SolutionA: 1 gram of salt in 1 m^(3) of solution has concentration of 1 ppm. R: ppm is defined as number of parts by mass of solute per million parts of solution.
View SolutionQuestions|Parts Per Million (PPM) And Parts Per Billion (PPB)|Summary
View SolutionOne of your friends defines molality as follows. “It is the number of gram moles of the solute dissolved per litre of solution: Is it correct?
View SolutionOne of your friends defines molality as follows. “It is the number of gram moles of the solute dissolved per litre of solution: Can you help him to define molality?
View SolutionMole Fraction|Questions|Parts Per Million (PPM) / Parts Per Billion (PPB)|Questions|Summary
View SolutionWhat is the concentration in ppm, of a solution containing 50mg of Hg per litre?
View Solution

