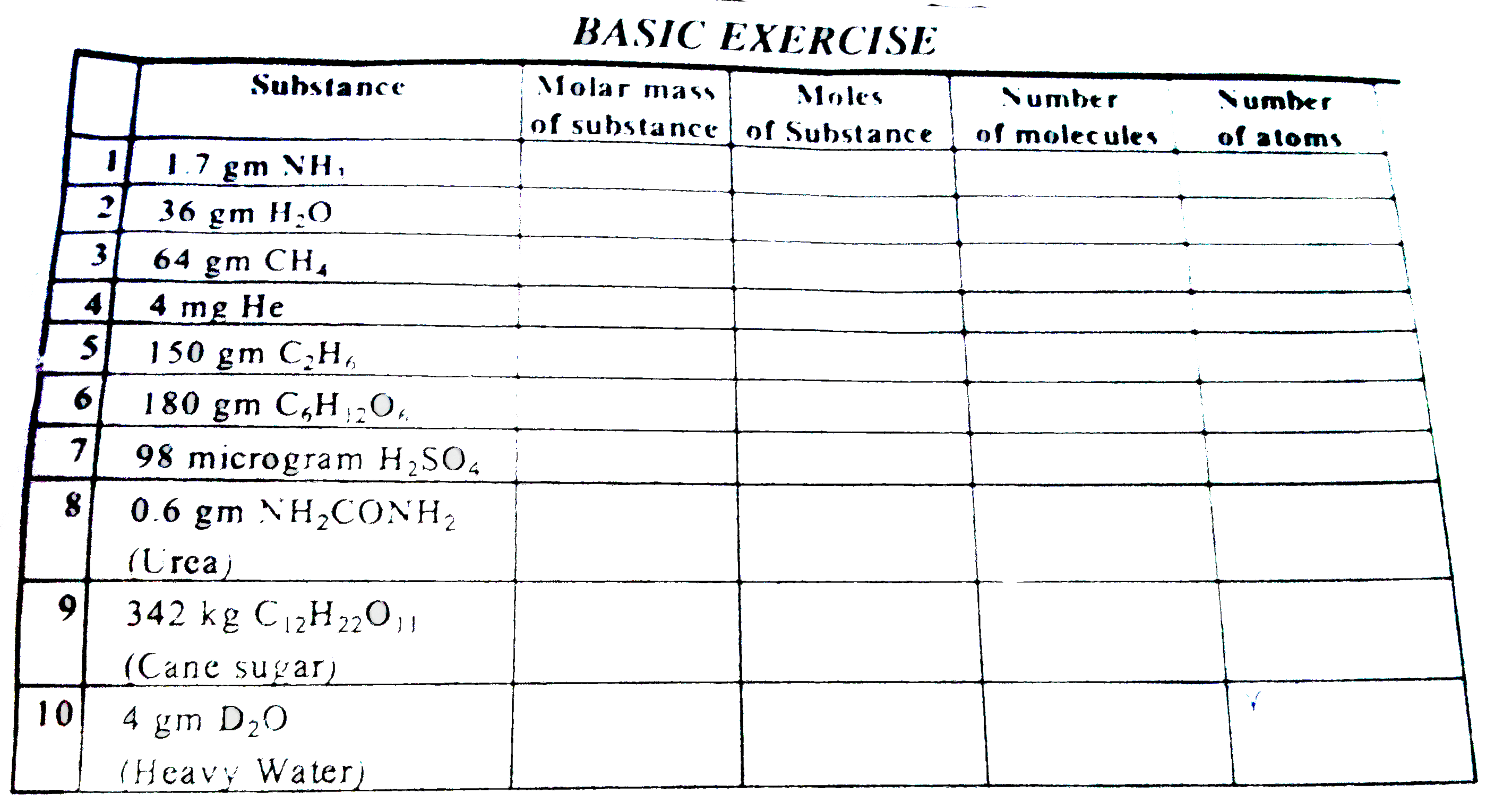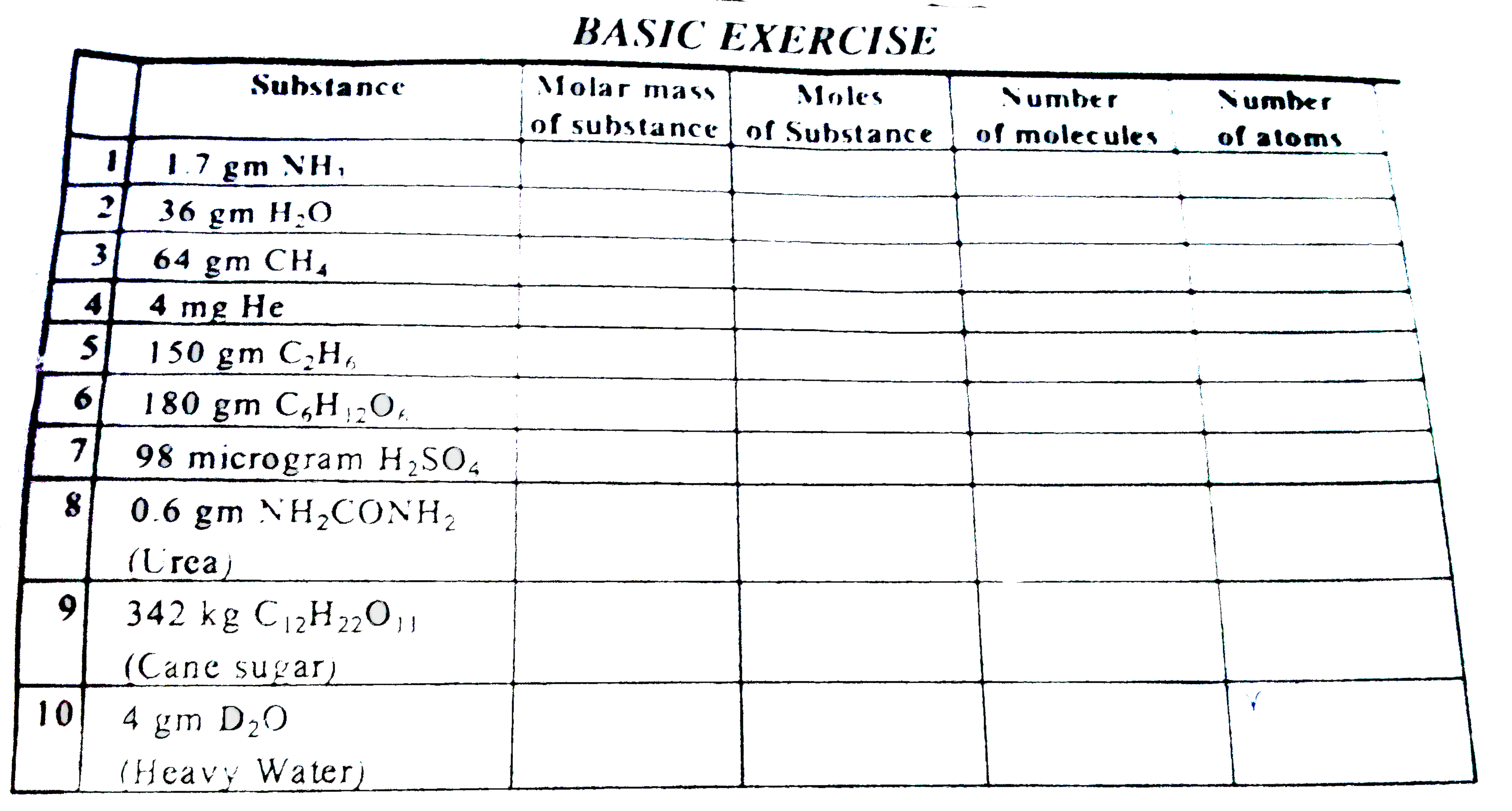
2 moles of H2SO4 is kept in a beaker. Find
(i) Moles of H-atoms
(ii) Moles of S-atoms
(iii) Moles of O-atoms
(iv) Number of O-atoms
The correct Answer is:(##ALNNCCHMMCE01003_A01##)
(i) 4 (ii) 2 (iii) 8 (iv) 8N4
Similar Questions
Knowledge Check
The number of carbon atoms in exactly 12 g C12 is called Avogadro's number , NA(6.022×1023) . One mole is the amount of material which contains Avogradro's number of particle.
These definitions emphasize that the mole refers to a fixed number of NA+ b Avogadro's number of atoms , electrons to refer to a mole of helium , a mole of electrons , or a mole of NA+ by Avogadro's number of atoms, electrons or ions respectively. On the other hand, phrases like "one mole of hydrogen" can be ambiguous , and should be restated as "one mole of hydroden atoms" or " one mole fo hydrogen molecules." But it is a matter of common practice among chemists , however , to let the name of the element stand for its mos common form. Thus one mole of O2 is frequently referred to as one mole of oxygen , whereas one moles of O is called one mole of oxygen atoms. Suppose if we take one mole molecules fo H2SO4 then it contain 2 moles of H atom 1 mole of S atom and 4 moles of O atom. It can also be said that moles of oxygen molecules is 42 =2 mole.
If a sample of CuSO4.5H2O contains 3 moles of oxygen molecules , then fo H-atom present in the sample is :A5B9C103D203The number of carbon atoms in exactly 12 g C12 is called Avogadro's number , NA(6.022×1023) . One mole is the amount of material which contains Avogradro's number of particle.
These definitions emphasize that the mole refers to a fixed number of NA+ b Avogadro's number of atoms , electrons to refer to a mole of helium , a mole of electrons , or a mole of NA+ by Avogadro's number of atoms, electrons or ions respectively. On the other hand, phrases like "one mole of hydrogen" can be ambiguous , and should be restated as "one mole of hydroden atoms" or " one mole fo hydrogen molecules." But it is a matter of common practice among chemists , however , to let the name of the element stand for its mos common form. Thus one mole of O2 is frequently referred to as one mole of oxygen , whereas one moles of O is called one mole of oxygen atoms. Suppose if we take one mole molecules fo H2SO4 then it contain 2 moles of H atom 1 mole of S atom and 4 moles of O atom. It can also be said that moles of oxygen molecules is 42 =2 mole.
Sulphur exists in different allotropic forms like S2S6'andS8 etc. If equal weight of these three ar taken in separate containers , then the ratio of number of atoms present in them is :A1:3:4B1:1:1C2:4:3D1:1:4The number of carbon atoms in exactly 12 g C12 is called Avogadro's number , NA(6.022×1023) . One mole is the amount of material which contains Avogradro's number of particle.
These definitions emphasize that the mole refers to a fixed number of NA+ b Avogadro's number of atoms , electrons to refer to a mole of helium , a mole of electrons , or a mole of NA+ by Avogadro's number of atoms, electrons or ions respectively. On the other hand, phrases like "one mole of hydrogen" can be ambiguous , and should be restated as "one mole of hydroden atoms" or " one mole fo hydrogen molecules." But it is a matter of common practice among chemists , however , to let the name of the element stand for its mos common form. Thus one mole of O2 is frequently referred to as one mole of oxygen , whereas one moles of O is called one mole of oxygen atoms. Suppose if we take one mole molecules fo H2SO4 then it contain 2 moles of H atom 1 mole of S atom and 4 moles of O atom. It can also be said that moles of oxygen molecules is 42 =2 mole.
According to common practise , if you have Avogadro number of hydrogen molecules, then its mass is equal to :A1 gmB2 gC4 gDdepends on temperature
In three moles of ethane(C2H6), calculate the following:
(i) Number of moles of carbon atoms.
(ii) Number of moles of hydrogen atoms.
(iii) Number of molecules of ethane.
View SolutionIn three moles of ethane(C2H6), calculate the following:
(i) Number of moles of carbon atoms.
(ii) Number of moles of hydrogen atoms.
(iii) Number of molecules of ethane.
View SolutionIn three moles of ethane(C2H6), calculate the following:
(i) Number of moles of carbon atoms.
(ii) Number of moles of hydrogen atoms.
(iii) Number of molecules of ethane.
View SolutionThe number of carbon atoms in exactly 12 g C12 is called Avogadro's number , NA(6.022×1023) . One mole is the amount of material which contains Avogradro's number of particle.
These definitions emphasize that the mole refers to a fixed number of NA+ b Avogadro's number of atoms , electrons to refer to a mole of helium , a mole of electrons , or a mole of NA+ by Avogadro's number of atoms, electrons or ions respectively. On the other hand, phrases like "one mole of hydrogen" can be ambiguous , and should be restated as "one mole of hydroden atoms" or " one mole fo hydrogen molecules." But it is a matter of common practice among chemists , however , to let the name of the element stand for its mos common form. Thus one mole of O2 is frequently referred to as one mole of oxygen , whereas one moles of O is called one mole of oxygen atoms. Suppose if we take one mole molecules fo H2SO4 then it contain 2 moles of H atom 1 mole of S atom and 4 moles of O atom. It can also be said that moles of oxygen molecules is 42 =2 mole.
From a mixture of 4 moles Ca3(PO4)2 , 5 moles of p4O10 and 6 moles of H3PO3 , all the phosphourus atoms are removed . Then moles of P4 molecules formed from all these atoms are:
View Solution2 moles of H atoms at NTP occupy a volume of:
View Solution