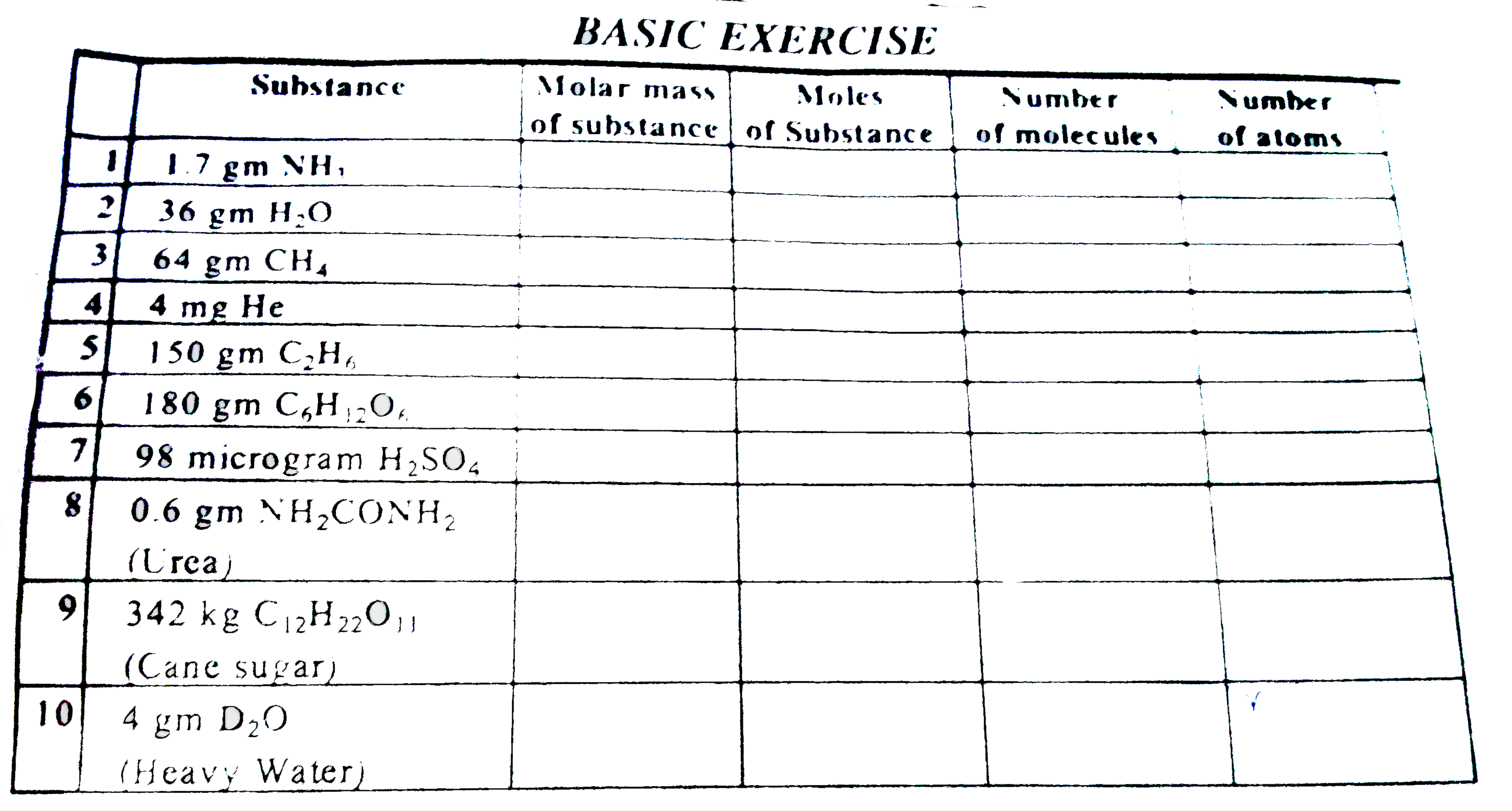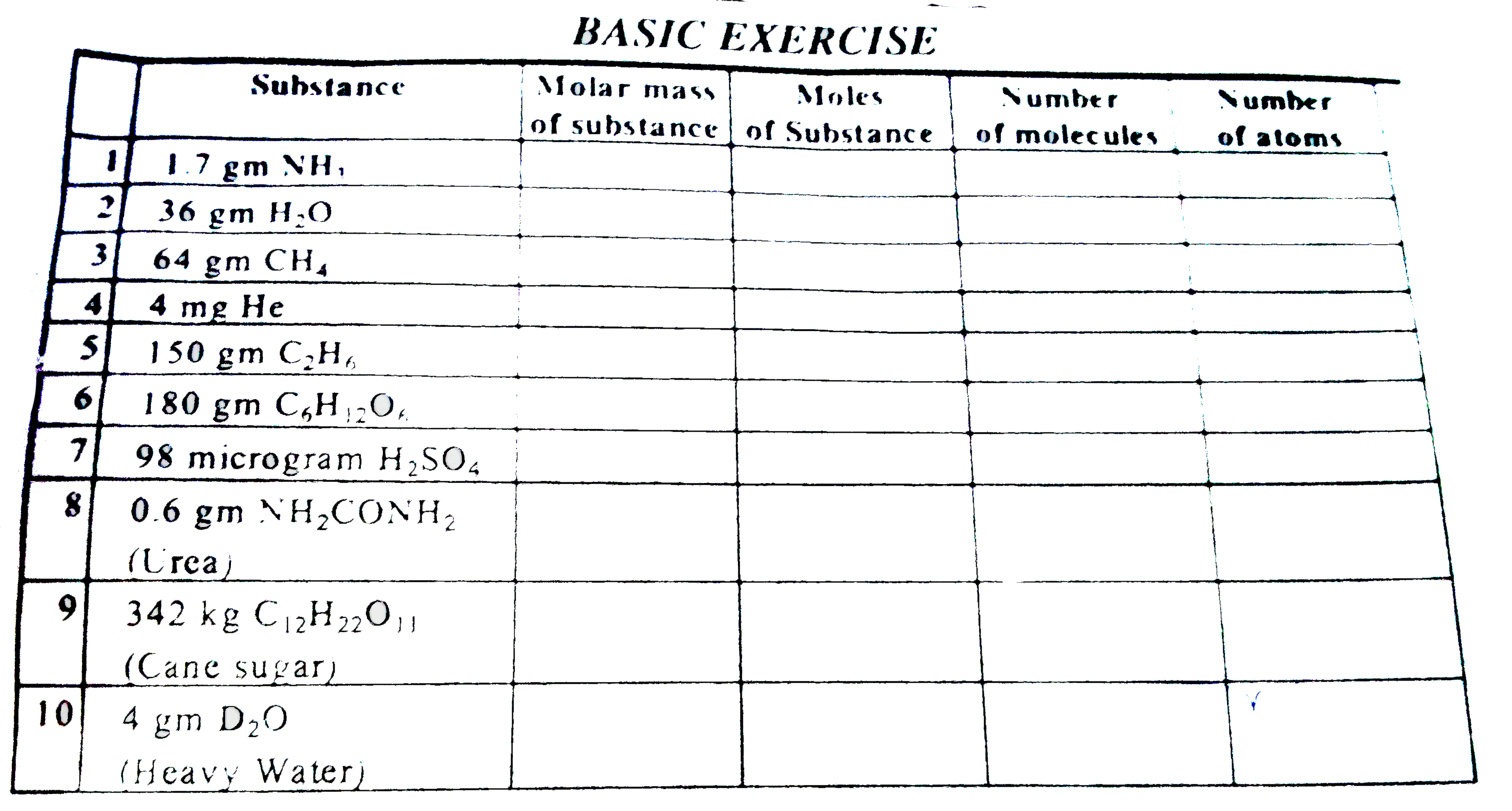
245 gm of KClO3 on heating yielded 64 gm O2.Find % yield of reaction.
(K = 39 , Cl = 35.5 )
The correct Answer is:(##ALNNCCHMMCE01027_A01##)
66.67
Similar Questions
Knowledge Check
Oxygen is prepared by catalytic decomposition of potassium chlorate (KClO3). Decomposition of potassium chlorate gives potassium chloride (KCl) and oxygen (O2). The following reaction takes place : 2KClO3(s)→2KCl(s)+3O2(g).
How many grams of KClO3 are required to produce 128 grams of O2? [At. mass of O = 16 u, K = 39 u, Cl = 35.5 u]A196 gB200 gC122 gD327 g
245g pure KClO3 decomposes to give 48g O2 .What is the % yield of reaction?
View SolutionWhat is mass of O2 required to produce 960gm of O3 if % yield of reaction 302→2O3 is 50% ? .
View SolutionWhat is mass of O2 required to produce 960gm of O3 if % yield of reaction 302→2O3 is 50% ? .
View SolutionO2 is evolved by heating KClO3 using MnO2 as a catalyst
2KClO3MnO2−−−→2KCl+3O2
Calculate the mass of KClO3 required to produce 6.72 litre of O2 at S.T.P. [atomic masses of K = 39, Cl = 35.5, O = 16).
View SolutionHow many grams of potassium chlorate should be decomposed to liberate 9.6 g of oxygen?
Equation: 2KClO3→2KCl+3O2
Relative atomic mass:K=39,Cl=35.5,O=16
View Solution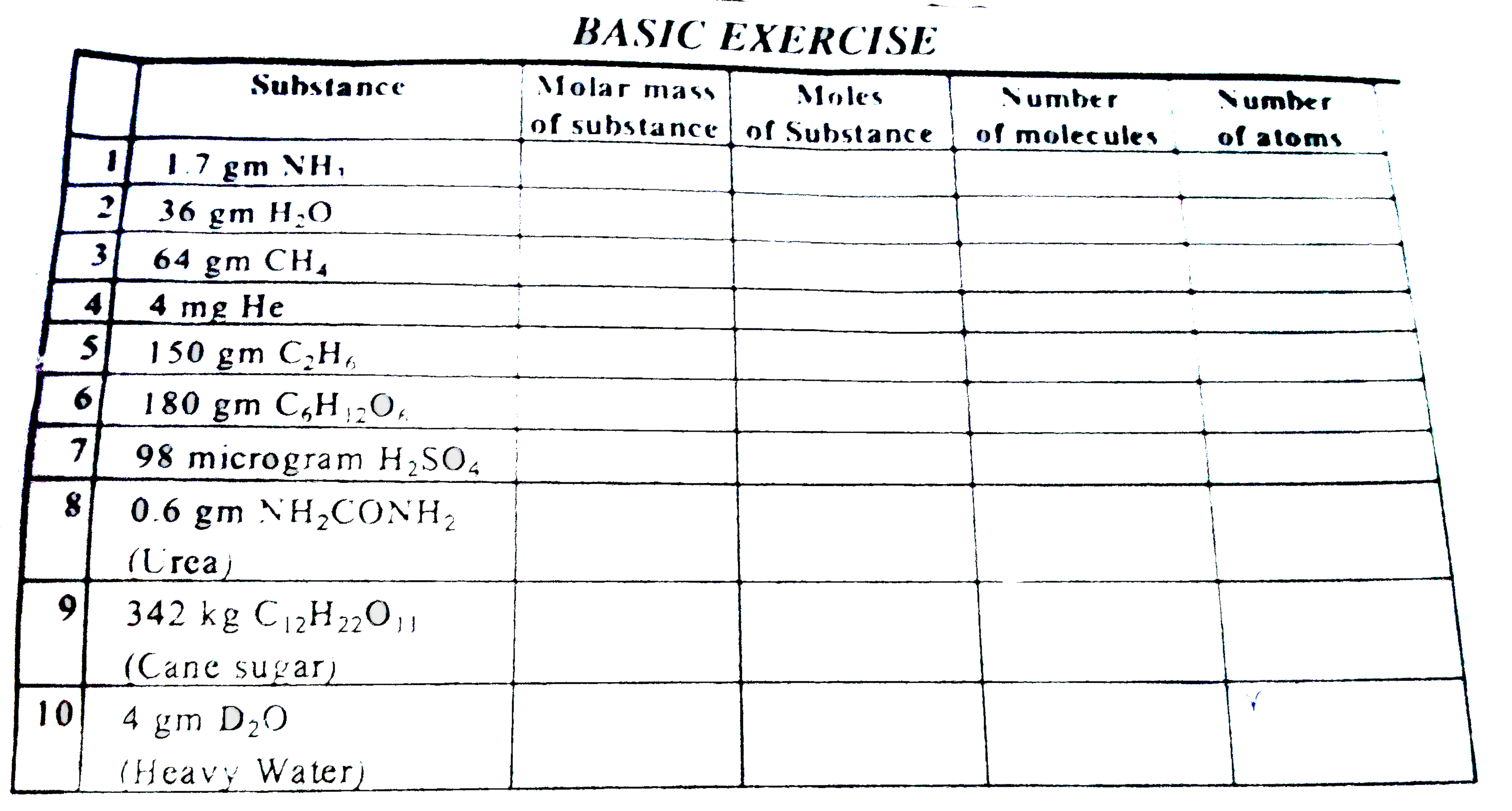
KClO3→KCl+O2(unbalanced),
If in above reaction 5 moles of KClO3 was heated, then find moles of O2 produced on completion of reaction.
View SolutionKClO3→KCl+O2(unbalanced),
If in above reaction 5 moles of KClO3 was heated, then find moles
of O2 produced on completion of reaction.
View Solution