The dipole moment of chlorobenzene is 1.6Dm The expected dipole moment of meta-dichlorobenzene is:
A
1.6D
B
3.2D
C
1.6√2D
D
0.0D
Video Solution
Text Solution
The correct Answer is:A
|
Answer
Step by step video, text & image solution for The dipole moment of chlorobenzene is 1.6Dm The expected dipole moment of meta-dichlorobenzene is: by Chemistry experts to help you in doubts & scoring excellent marks in Class 12 exams.
Updated on:21/07/2023Topper's Solved these Questions
Similar Questions
Knowledge Check
The dipole moment of chlorobenzene is 1.73 D . The dipole moment of p-dichlorobenzene is expected to be
A3.46 DB0.00DC1.73 DD1.00 DElectric dipole moment is
AscalerBneither scaler nor vectorCa vector directed from −q→+qDa vector directed from +q→−qDipole moment of cis-2, 3-dichloro-2 butne is ….. Than the dipole moment of cis1, 2-dichloroethene.
AmoreBlessCneither more nor lessDNone of the above
Dipole moment is maximum for
View SolutionThe dipole moment of o, p and m-dichlorobenzene will be in the order :
View SolutionThe dipole moment of o, p and m-dichlorobenzene will be in the order
View SolutionDipole moment of
 is 1.5D. The dipole moment of
is 1.5D. The dipole moment of
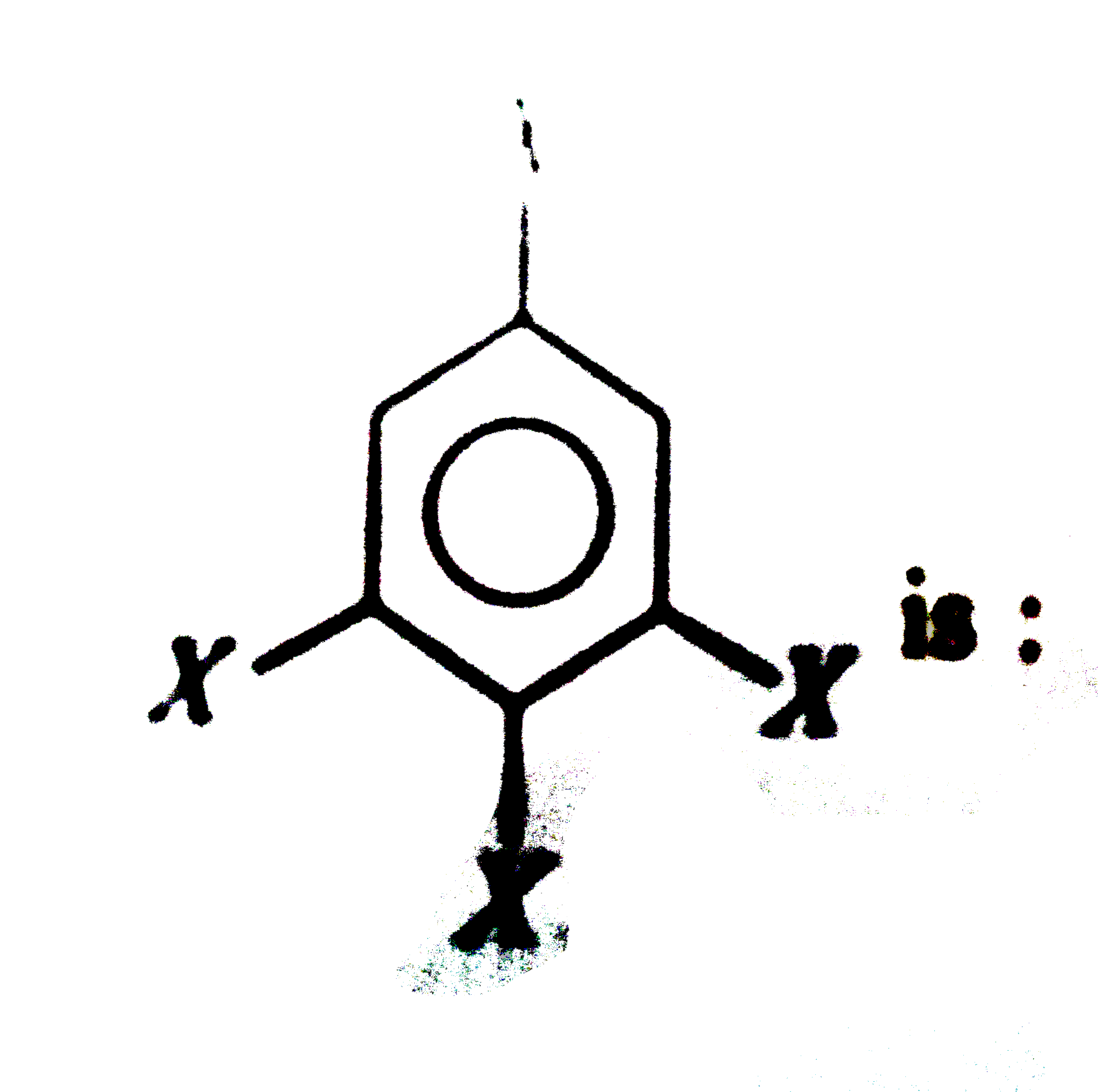
View SolutionThe dipole moment of o, p and m-dichlorobenzene will be in the order :
View Solution

