Draw the structure of diborane.
To draw the structure of diborane (B2H6), follow these steps:
Step 1: Understand the Molecular Formula
Diborane has the molecular formula B2H6, which indicates that it contains two boron (B) atoms and six hydrogen (H) atoms.
Hint: Remember that the subscripts in the molecular formula indicate the number of each type of atom present in the compound.
Step 2: Identify the Arrangement of Atoms
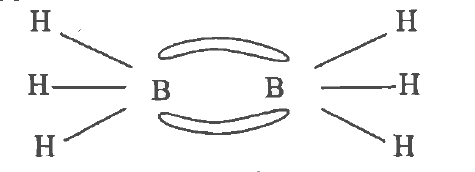
In diborane, the two boron atoms are central, and the hydrogen atoms are arranged around them. There are four terminal hydrogen atoms and two bridging hydrogen atoms.
Hint: Visualize how the boron atoms can be connected by the hydrogen atoms, keeping in mind the total number of hydrogen atoms.
Step 3: Draw the Terminal Hydrogen Atoms
Start by placing the two boron atoms in the center. Then, draw four terminal hydrogen atoms around each boron atom. Each boron atom will be connected to two terminal hydrogen atoms.
Hint: Each terminal hydrogen atom is bonded directly to a boron atom.
Step 4: Add the Bridging Hydrogen Atoms
Next, add the two bridging hydrogen atoms. These hydrogen atoms connect the two boron atoms together. They are positioned above and below the line connecting the two boron atoms.
Hint: The bridging hydrogen atoms create a three-dimensional structure, so think about how they connect the two boron atoms.
Step 5: Finalize the Structure
Now, ensure that the structure reflects the bonding correctly. Each boron atom should have an overall coordination of four, which includes the two terminal hydrogen atoms and the two bridging hydrogen atoms.
Hint: Check that each boron atom satisfies the bonding requirements and that the overall structure is balanced.
Final Structure
The final structure of diborane (B2H6) can be represented as follows:
HH B−BHH H
Summary
In summary, diborane (B2H6) consists of two boron atoms connected by two bridging hydrogen atoms, with four terminal hydrogen atoms attached to the boron atoms. The structure is unique due to the presence of these bridging hydrogen atoms, which contribute to the overall geometry of the molecule.
Diborane has the molecular formula B2H6, which indicates that it contains two boron (B) atoms and six hydrogen (H) atoms.
In diborane, the two boron atoms are central, and the hydrogen atoms are arranged around them. There are four terminal hydrogen atoms and two bridging hydrogen atoms.
Start by placing the two boron atoms in the center. Then, draw four terminal hydrogen atoms around each boron atom. Each boron atom will be connected to two terminal hydrogen atoms.
Next, add the two bridging hydrogen atoms. These hydrogen atoms connect the two boron atoms together. They are positioned above and below the line connecting the two boron atoms.
Now, ensure that the structure reflects the bonding correctly. Each boron atom should have an overall coordination of four, which includes the two terminal hydrogen atoms and the two bridging hydrogen atoms.
The final structure of diborane (B2H6) can be represented as follows:
In summary, diborane (B2H6) consists of two boron atoms connected by two bridging hydrogen atoms, with four terminal hydrogen atoms attached to the boron atoms. The structure is unique due to the presence of these bridging hydrogen atoms, which contribute to the overall geometry of the molecule.
Topper's Solved these Questions
- P-BLOCK ELEMENTSBOOK - CBSE COMPLEMENTARY MATERIALCHAPTER - P-BLOCK ELEMENTSEXERCISE - 3-MARK QUESTION
20 Videos
- P-BLOCK ELEMENTSBOOK - CBSE COMPLEMENTARY MATERIALCHAPTER - P-BLOCK ELEMENTSEXERCISE - 5-MARK QUESTION
16 Videos
- P-BLOCK ELEMENTSBOOK - CBSE COMPLEMENTARY MATERIALCHAPTER - P-BLOCK ELEMENTSEXERCISE - 1-MARK QUESTION
30 Videos
- ORGANIC CHEMISTRY: SOME BASIC PRINCIPLES AND TECHNIQUESBOOK - CBSE COMPLEMENTARY MATERIALCHAPTER - ORGANIC CHEMISTRY: SOME BASIC PRINCIPLES AND TECHNIQUESEXERCISE - Unit test
16 Videos
- SAMPLE PAPER - IV BOOK - CBSE COMPLEMENTARY MATERIALCHAPTER - SAMPLE PAPER - IV EXERCISE - SECTION-B
3 Videos
Similar Questions
Give reasons of the following
In diborane, two B - H - B bonds are different from common covalent bonds.
View SolutionWhen metal X is treated with sodium hydroxide, a white precipitate (A) is obtained, which is soluble in excess of NaOH to give soluble complex (B). Compound (A) is soluble in dilute HCl to form compound (C). The compound (A) when heated strongly gives (D), which is used to extract metal. Identify (X), (A), (B), (C) and (D). Write suitable equations to support their identities.
View SolutionMention two important ores of boron.
View SolutionWrite bond line formula for the following compound HOCH2CH2CH(CH3)CH(CH3)COOH
View Solution0.40 g of an organic compound gave 0.30 g of silver bromide by carius method. Determine the percentage bromine in the compound.
View SolutionIn the structure of diborane
View SolutionIn the structure of diborane,
View SolutionIn the structure of diborane
View SolutionGive the structure of diborane.
View SolutionStructure of diborane.
View Solutionडाइबोरेन क्या है ? इसे बनाने की विधि लिखिए। इसकी संरचना चित्र बनाकर समझाइए।
View SolutionIn the structure of diborane :
View Solutionडाइबोरेन की संरचना में-
View Solutionडाइबोरेन की संरचना में-
View SolutionIn the structure of diborane,
View Solution

