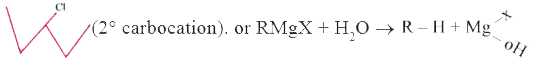Out of 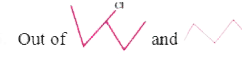 which undergo SN1 reaction faster and why?
which undergo SN1 reaction faster and why?
Why grignard reagent should be prepared under anhydrous conditions?
Topper's Solved these Questions
- PRACTICE - PAPERBook:CBSE COMPLEMENTARY MATERIALChapter:PRACTICE - PAPERExercise:Section : B
Explore 7 Videos
- PRACTICE - PAPERBook:CBSE COMPLEMENTARY MATERIALChapter:PRACTICE - PAPERExercise:Section : C
Explore 6 Videos
- POLYMERS Book:CBSE COMPLEMENTARY MATERIALChapter:POLYMERS Exercise:LONG ANSWER TYPE QUESTIONS
Explore 5 Videos
- PRACTICE PAPER 1Book:CBSE COMPLEMENTARY MATERIALChapter:PRACTICE PAPER 1Exercise:Questions
Explore 82 Videos
Similar Questions
Knowledge Check
Grignard reagent should be prepared under anhydrous conditions because
Ait is highly reactive towards protonic substancesBit reacts with etherCboth (a) and (b)Dnone of the aboveWhich will undergo SN2 reaction faster
A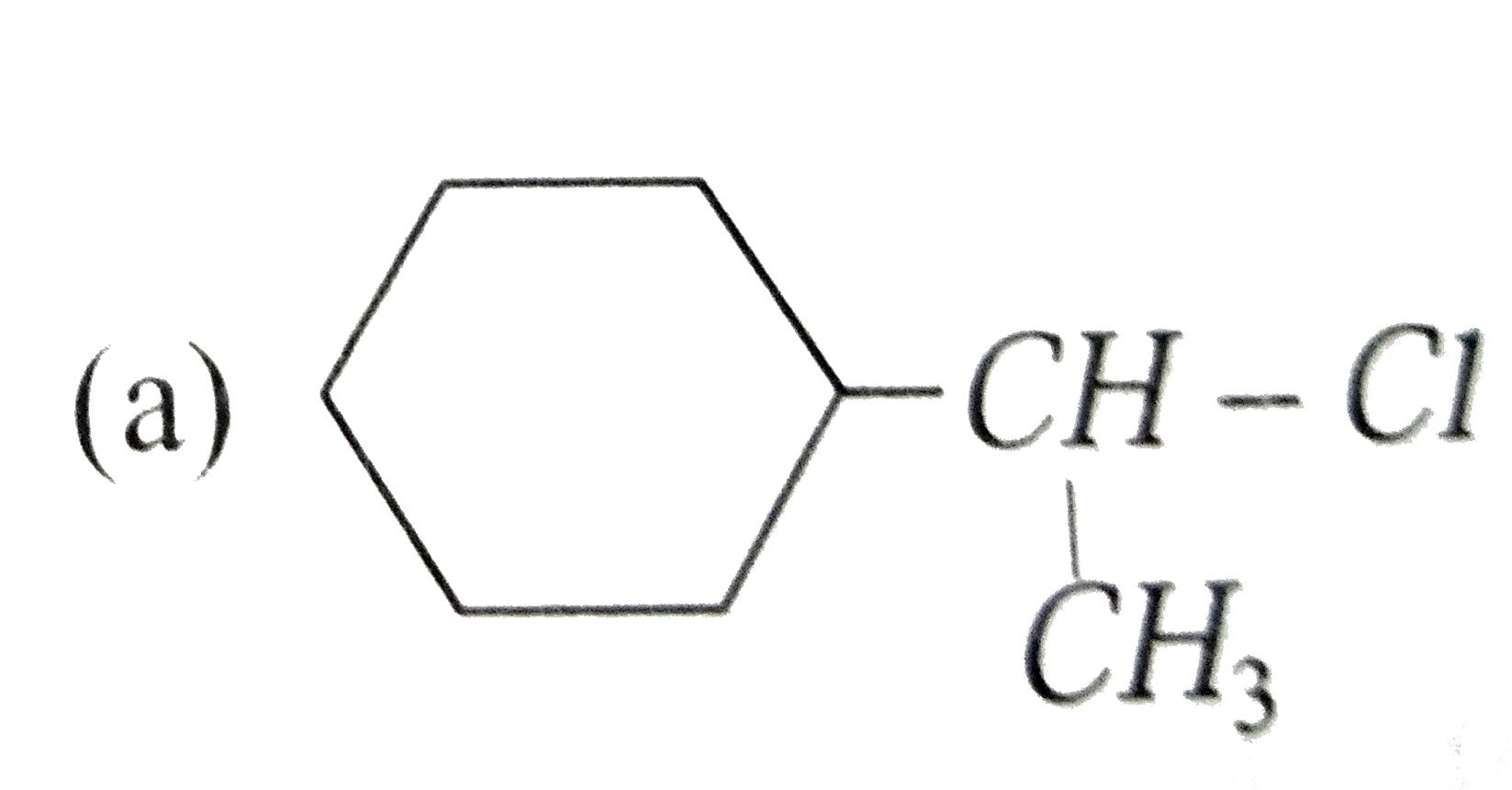 B
B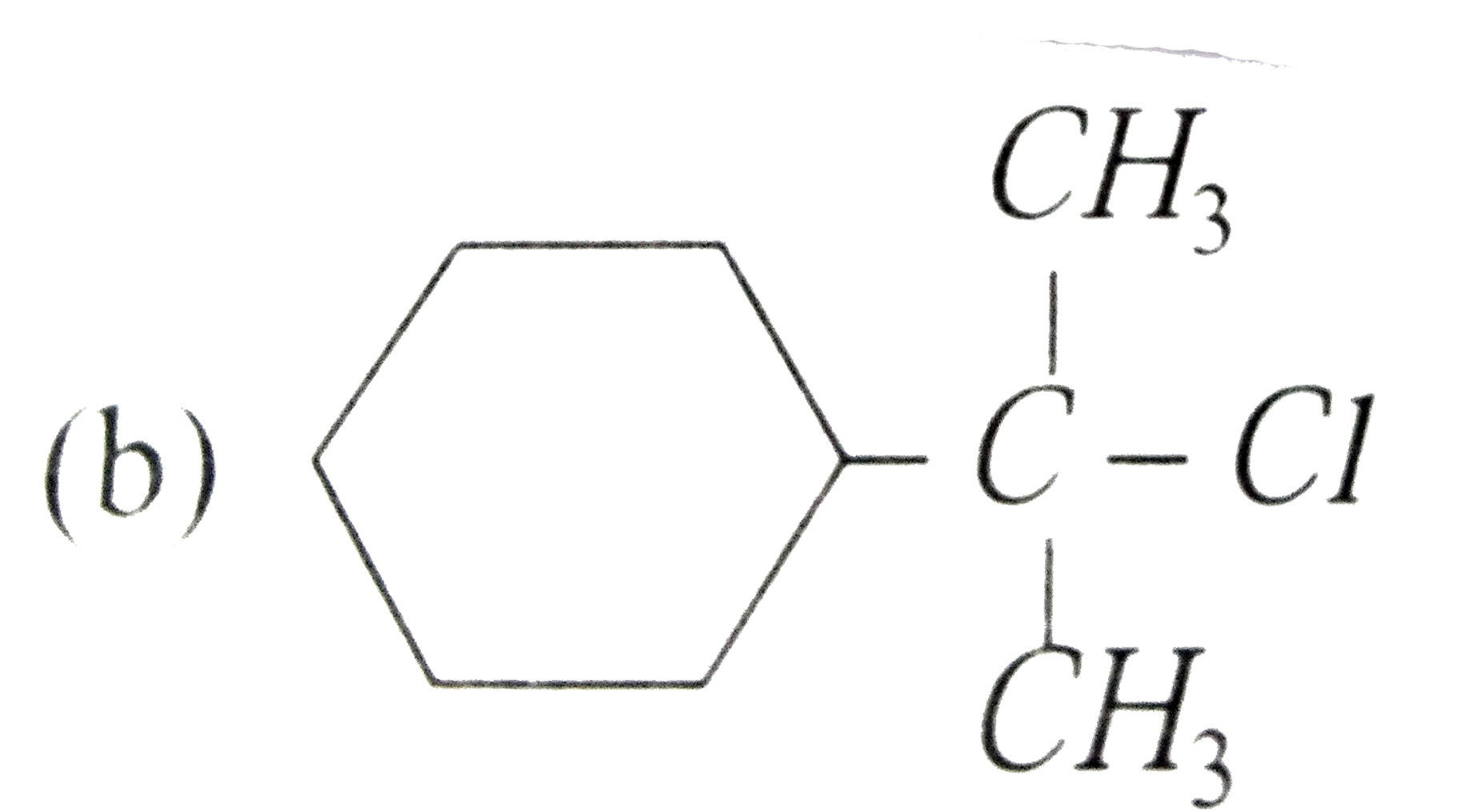 C
C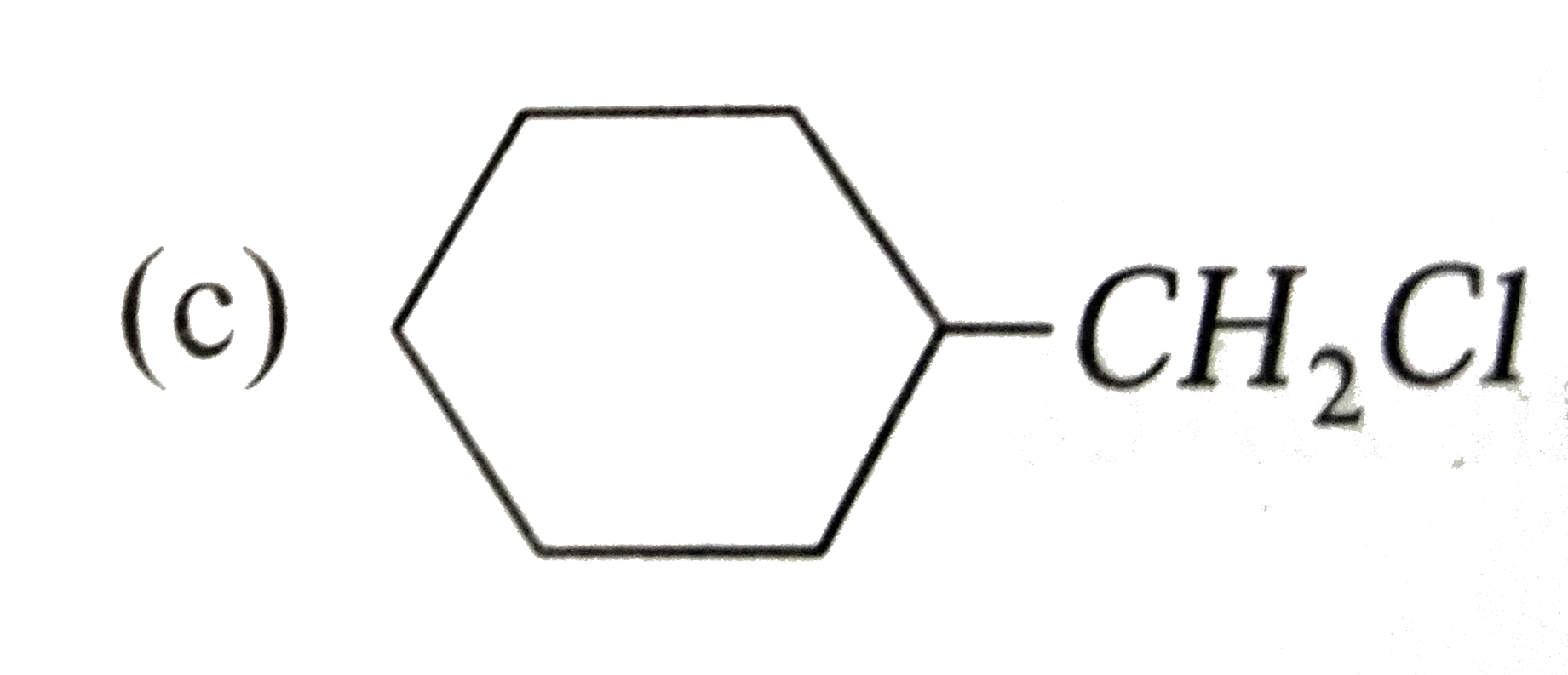 D
D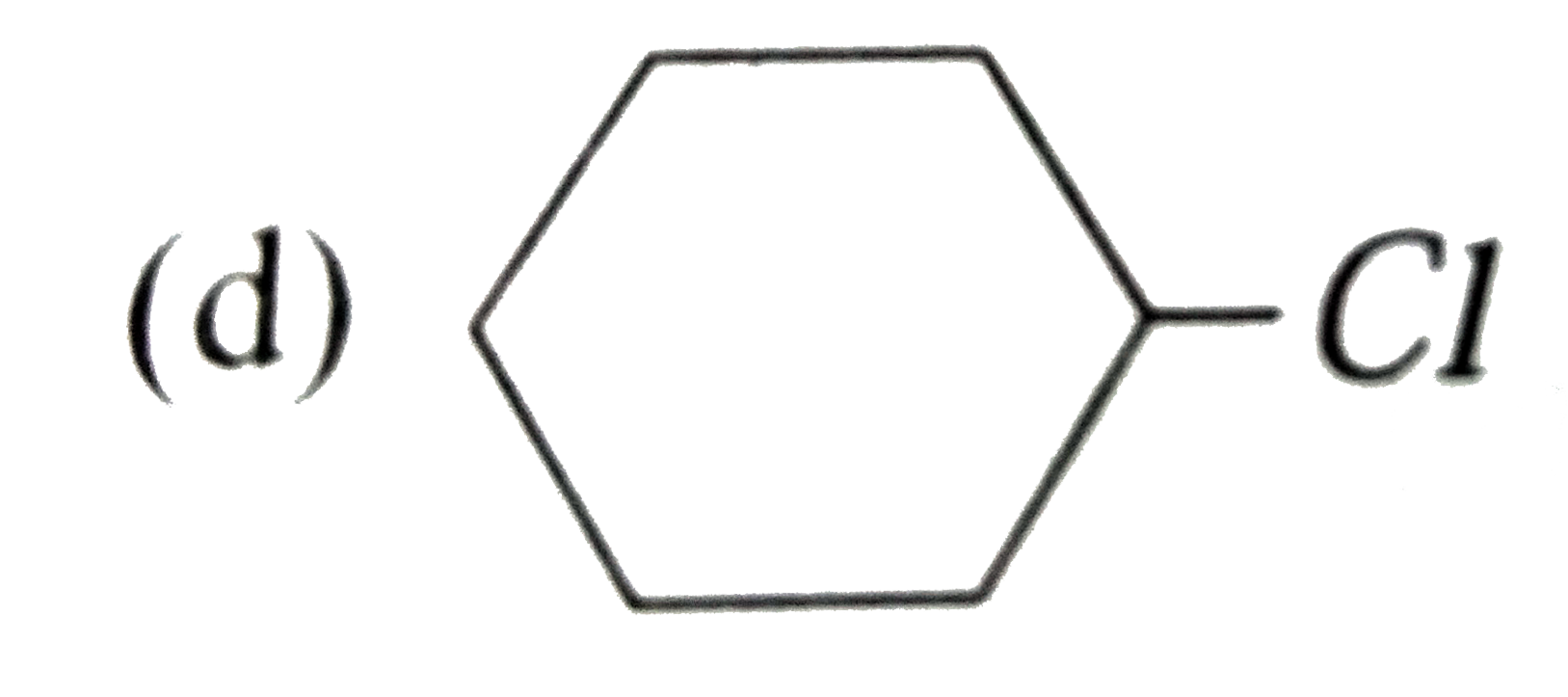
Explain :
Grignard reagents should be prepared under anhydrous conditions.
View SolutionExplain why
(a) the dipole moment of chlorobenzene is lower than that of cyclohexyl chloride ?
(b) alkyl halides, though polar, are immiscible with water ?
(c) vinyl chloride is unreactive in nucleophilic substitution reactions ?
(d) neopentyl bromide undergoes nucleophilic substitution reactions very slowly ?
(e) 3-bromocyclohexane is more reactive than 4- bromocyclohexane in hydrolysis with aqueous NaOH ? (f) tert butyl chloride reacts with aqueous sodium hydroxide by SN1 mechanism while n-butyl chloride reacts by SN2 mechanism.
(g) Grignard reagents should be prepared under anhydrous conditions ?
View SolutionExplain why
(i) the dipole moment of chlorobenzene is lower than that of cyclohexyl chloride?
(ii) alkyl halides, though polar, are immiscible with water?
(iii) Grignard reagents should be prepared under anhydrous conditions?
View SolutionExplain why
(i) the dipole moment of chlorobenzene is lower than that of cyclohexyl chloride?
(ii) alkyl halides, though polar, are immiscible with water?
(iii) Grignard reagents should be prepared under anhydrous conditions?
View SolutionWhat is Grignard reagent? How is it prepared? Why are they prepared under anhydrous condition?
View SolutionExplain: (i) Dipole moment of chlorobenzene is lower than that of cyclohexylchloride
(ii). Alkyl halide though polar, are immiscible with water.
(iii). Grignard reagents should be prepared under anhydrous conditions.
View Solution