For the sysnthesis of ammonia,
N2(g)+3H2(g)→2NH3(g)
Calculate ΔH∘,ΔS∘andΔG∘ at 300 K using the following data:
SpeciesN2H2NH3ΔH∘f(kJ mol−1)00−46.2S∘(JK−1 mol−1)191.5130.6192.5
ΔH∘=−92.4kJ mol−1,ΔS∘=−198.3JK−1mol−1
ΔG∘=−32.91kJ mol−1
Similar Questions
For the synthesis of ammonia at 300 K :
N2(g)+3H2(g)→2NH3(g)
Calculate the value of ΔG∘ in Kcal and give your answer in magnitude by using the following data :
()N2H2NH3ΔHf(Kcal/mole)00−10S∘(Cal/K-mole)403045
View SolutionFor the formation of C(g)at300 K.
A(g)+3B(g)→2C(g)
Calculate the magnitude of ΔG∘ (Kcal) if given data:
ABCΔH∘f(Kcal mol−1)00−10ΔS∘f(Cal K−1mol−1)403045
View Solution
Knowledge Check
For the reaction
N2(g)+3H2(g)→2NH3(g), which of the following is correct regarding ΔH ?AΔH=ΔU+RTBΔH=ΔU+2RTCΔH=ΔU−2RTDΔH=ΔU−RTThe % yield of ammonia as a function of time in the reaction N2(g)+3H2(g)⇔2NH3(g),(g)ΔH<0 at (P,T1) is given below
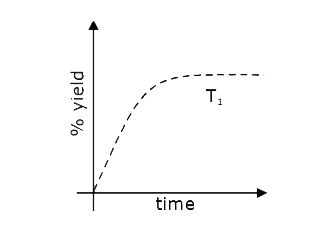 A
A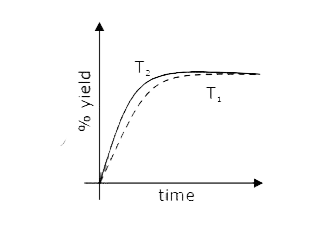 B
B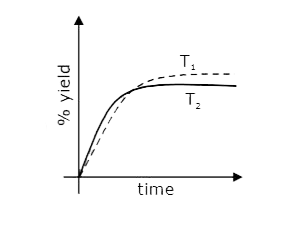 C
C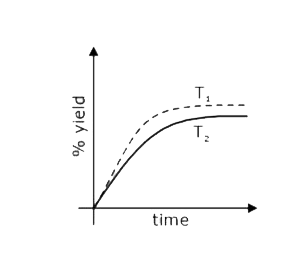 D
D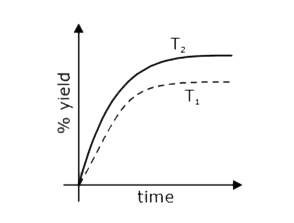
For the reaction N2(g)+3H2(g)→2NH3(g),ΔH=−93.6KJmol−1 the formation of NH3 is expected to increase at :
AHigh pressure and low temperatureBLow pressure and low temperatureCHigh pressure and high temperatureDLow pressure and high temperature
The heat change for the reaction,
N2(g)+3H2(g)→2NH3(g)
is -92.2kJ. Calculate the heat of formation of ammonia.
View SolutionGiven:
N2(g)+3H2(g)→2NH3(g),ΔrH∘=−924kJmol−1. What is the standard enthalpy of formation of NH3 gas?
View SolutionGiven
N2(g)+3H2(g)→2NH3(g),ΔrHӨ=−92.4kJmol−1
What is the standard enthalpy of formation of NH3 gas?
View SolutionFor the reaction N2(g)+3H2(g)⇔2NH3(g),ΔH=−93.6kJmol−1. The concentration of H2 at equilibrium will increase if
View SolutionThe standard entropies of N2(g),H2(g) and NH3(g) are 191.5,192.6JK−1mol−1 . The value of ΔS0 during the formation of 1 mole of ammonia is
View Solution
