For the reversible process, the value of ΔS is given by the expression:
qrevT
T−qrev
qrev×T
qrev−T
The correct Answer is:A
Step by step video, text & image solution for For the reversible process, the value of DeltaS is given by the expression: by Chemistry experts to help you in doubts & scoring excellent marks in Class 12 exams.
Updated on:21/07/2023Topper's Solved these Questions
- Chemical Thermodynamics and ThermochemistryBOOK - OP TANDONCHAPTER - Chemical Thermodynamics and ThermochemistryEXERCISE - Set 2
18 Videos
- Chemical Thermodynamics and ThermochemistryBOOK - OP TANDONCHAPTER - Chemical Thermodynamics and ThermochemistryEXERCISE - Objective Question Level B
56 Videos
- Chemical Thermodynamics and ThermochemistryBOOK - OP TANDONCHAPTER - Chemical Thermodynamics and ThermochemistryEXERCISE - Practice problems
81 Videos
- CHEMICAL KINETICSBOOK - OP TANDONCHAPTER - CHEMICAL KINETICSEXERCISE - LINKED COMPRESHENSION TYPE QUESTIONS ( SECTION-VI)
4 Videos
- CLASSIFICATION AND NOMENCLATURE OF ORGANIC COMPOUNDS BOOK - OP TANDONCHAPTER - CLASSIFICATION AND NOMENCLATURE OF ORGANIC COMPOUNDS EXERCISE - INTEGER
2 Videos
Similar Questions
Which is intensive property ?
View SolutionIn which of the following pairs, both proeperties are intensive?
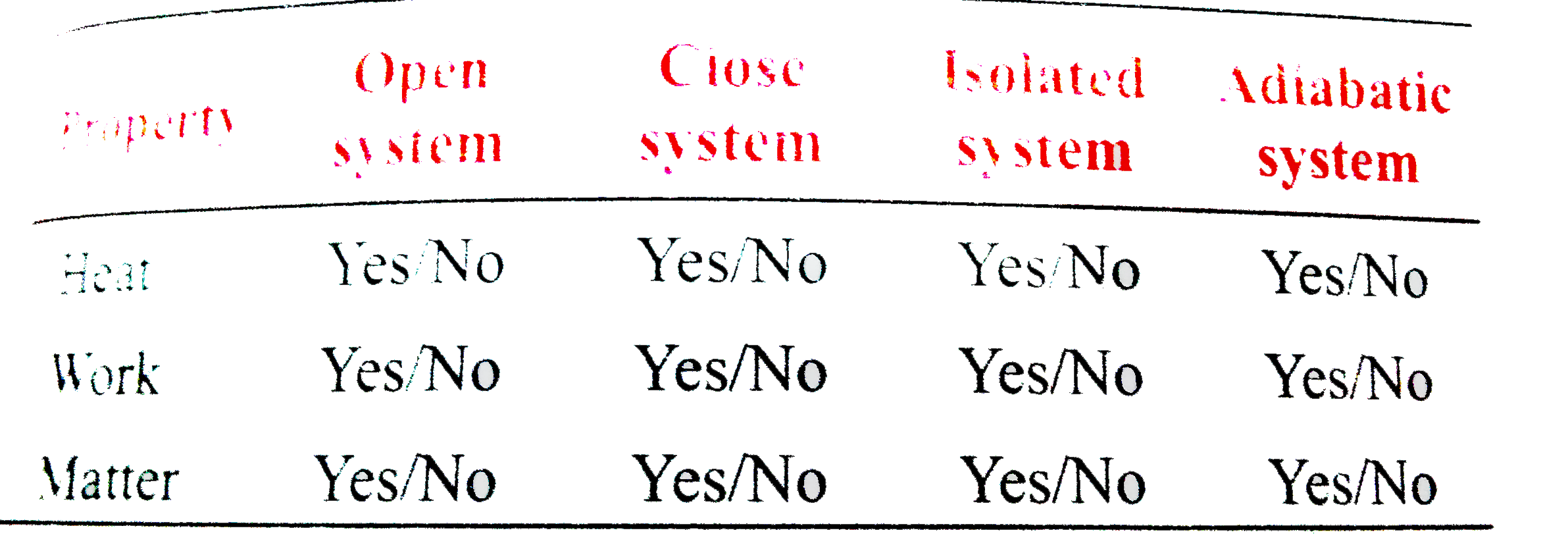
View SolutionWrite 'yes' if heat, work or matter are able to cross the boundary of the corresponding system and 'no' if passage is forbidden:
View SolutionThe rate of chemical reaction are strongly affected by temperature . Arrhenius (1889) gave following relation between rate contant and temperature .
k=Ae−Ea/RT .....(i)
This equation is called Arrhenius equation. The constant 'A' is caleld Arrhenius of pre-exponential factor. Logarithm of equation (i) given :
log10k=log10AEa2.3030RT .....(ii)
The rate constant for a reaction is .
log10sec−1=14−1.25×104KT .....(iii)
The pre-exponential factor or frequency factor of the reaction under consideration is :
View SolutionThe total number of alkyl groups having the molecular formula (c)C3H7(b)C4H9(c)C5H11 are.
View SolutionFor the reversible process, the value of ΔS is given by the expression:
View SolutionGiven two examples of reversible processes. Discuss their reversibility.
View SolutionFor reversible process at equilibrium, the change in entropy may be expressed as
View SolutionFor a reversible isothermal process in equilibrium, the entropy change is given by the expression
View SolutionFor an isothermal reversible expansion process, the value of q can be calculated by the expression
View SolutionIn a reversible process, the value of Delta S _(sys) + Delta S_(surr) is
View SolutionFor a reversible process at equilibrium, the change in entropy may be expressed as:
View Solutionउत्क्रमणीय प्रक्रम द्वारा किया गया अधिकतम कार्य निम्न द्वारा व्यक्त किया जाता है
View Solutionसमतापीय उत्क्रमणीय प्रसार प्रक्रम के लिए, q के मान की गणना किस व्यंजक द्वारा की जा सकती है?
View SolutionFor an isothermal reversible phase transition process , ΔS is
View SolutionFor an isothermal reversible phase transition process , ΔS is
View Solution

