The approxiamte standard enthalpies of formation of methanol and octane are determiend to be -1.5 kJ/mol and -10.9 kJ/mol respectively. The standard enthalpies of combustion of octane is denoted as ΔH(octane) and that fo methanol as ΔH (methanol). the correct statement is:
ΔH (octane) is more negative than ΔH (methanol)
ΔH(octane) is less negative than ΔH (methanol)
ΔH (octane) is equal to ΔH (methanol)
ΔH (octane)+ΔH(methanol)=0
The correct Answer is:A
Heat of combustion of octane will be more negative than methanol because octane has greaterr number of carbon than methanol.
Similar Questions
Knowledge Check
The enthalpies of combustion of carbon and carbon monoxide are −390kJmol−1 and −278kJmol−1 respectively. The enthalpy of formation of carbon monoxide is
A668kJmol−1B112kJmol−1C−112kJmol−1D−668kJmol−1The enthalpies of formation of CO and CO2 are −110.5KJmol−1 and −393.5KJmol−1 respectively. The enthalpy of combustion of carbon monoxide is
A−110.5KJmol−1B−283.0KJmol−1C504.0KJmol−1D221.2KJmol−1The standard enthalpies of formation of CO2(g), H2O(?) and glucose(s) at 25∘C are -400kJ/mol, -300kJ/mol and -1300 kJ/mol, respectively. The standard enthalpy of combustion per gram of glucose at 25∘C is
A+2900kJB−2900kJC−16.11kJD+16.11kJ
Calculate the standard enthalpy of formation of liquid methanol from the following data
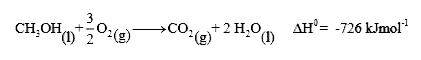
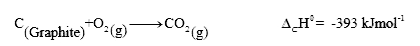

View SolutionThe standard enthalpies of formation of CO2(g),H2O(l) and glucose (s) at 25∘C are −400 kJ/mol, −300 kJ/mol and −1300 kJ/mol, respectively. The standard enthalpy of combustion per gram of glucose at 25∘C is :
View SolutionThe standard enthalpies fo formation of CO2(g),H2O(1), and glucose (s) at 25∘C are −400kJmol−1,−300kJmol−, and −1300kJmol−1, respectively. The standard enthalply of combustion per gram of glucose at 25∘C is
View SolutionIf standard enthalpy of vapourisation of wate is 42 kJ/mol, then -
View SolutionThe standard enthalpies of formation of CO2(g),H2O(l)and glucose(s) at 25∘C are -400 kJ/mol. -300kJ/mol and -1300 kJ/mol, respectively. The standard enthalpy of cumbusion per gram of glucose at 25∘C is
View Solution
