Prove that for two half reactions having potentials E1 and E2 which are combined to yield a third half reaction, having a potential E3,
E3=n1E1+n2E2n3
ΔG3=ΔG1+ΔG2
−n3FE3=−n1FE1−n2FE2
or n3E3=n1E1+n2E2
or E3=n1E1+n2E2n3
Topper's Solved these Questions
- ELECTROCHEMISTRYBook:OP TANDONChapter:ELECTROCHEMISTRYExercise:ILLUSTRATIONS
Explore 28 Videos
- ELECTROCHEMISTRYBook:OP TANDONChapter:ELECTROCHEMISTRYExercise:PRACTICE PROBLEMS
Explore 94 Videos
- COORDINATION COMPOUNDSBook:OP TANDONChapter:COORDINATION COMPOUNDSExercise:SBJECTIVE TYPE
Explore 96 Videos
- HALOALKANES AND HALOARENES Book:OP TANDONChapter:HALOALKANES AND HALOARENES Exercise:Single Interger answer type questions
Explore 14 Videos
Similar Questions
Statement : If two half reaction with electrode potential E∘1 and E∘2 gives a third reaction then,
ΔG∘3=ΔG∘1+ΔG∘2
Explanation : E∘3=E∘1+E∘2
View SolutionThree coins are tossed. Let E1 denotes "Three heads or three tails" E2, denotes ''at least two heads'' and E3, denotes'' at most two tails''. Which of the following pairs (E1,E2),(E1,E3)and(E2,E3) arindependent and which are not ?
View Solution
Knowledge Check
Statement : If two half reaction with electrode potential E∘1 and E∘2 gives a third reaction then,
ΔG∘3=ΔG∘1+ΔG∘2
Explanation : E∘3=E∘1+E∘2ASTATEMENT-1 is True, STATEMENT-2 is True, STATEMENT -2 is correct explanation for STATEMENT-1BSTATEMENT 1 is True, STATEMENT-2 Is True, STATEMENT -2 is NOT a correct explanation for STATEMENT-1CSTATEMENT - 1 is True, STATEMENT-2 is FalseDSTATEMENT-1 is False, STATEMENT-2 is Truewhen two half-cells of electrode potential of E1 and E2 are combined to form a half cell of electrode potential E3 , then
(when n1,n2andn3 are no. of electrons exchanged in first second and combined half-cells:AE3=E2−E1BE3=E1n1+E2n2n3CE3=E1n1+E2n2n23DE3=E1−E2Read the following passage for the evaluation of E∘ when different number of electrons are involved. Consider addition of the following half reactions
(1) Fe3+(aq)+3e−→Fe(s)E∘1=0.45V
(2) Fe(s)→Fe2+(aq)+2e−E∘2=−0.04V
(3) Fe3+(aq)+e−→Fe2+(aq)E∘3=?
Because half-reactions (1) and (2) contains a different number of electrons, the net reaction (3) is another half-reaction and E∘3 can't be obtained simply by adding E∘1 and E∘3. The free - energy changes however, are additive because G is a state function : ΔG∘3=ΔG∘1+ΔG∘2
If number of electrons in the reacton are n1,n2 and n2 respectively, then standard electrode potential of reation (3) isAE∘3=n1E∘1+n2E∘2n3BE∘3=n1E∘1−n2E∘2n3CE∘3=(E1−E2)D−E∘3=(E1+E2)
If E1andE2 are two events and E2 is a subset of E1 then P(E1/E2)=_____
View SolutionRead the following passage for the evaluation of E∘ when different number of electrons are involved. Consider addition of the following half reactions
(1) Fe3+(aq)+3e−→Fe(s)E∘1=0.45V
(2) Fe(s)→Fe2+(aq)+2e−E∘2=−0.04V
(3) Fe3+(aq)+e−→Fe2+(aq)E∘3=?
Because half-reactions (1) and (2) contains a different number of electrons, the net reaction (3) is another half-reaction and E∘3 can't be obtained simply by adding E∘1 and E∘3. The free - energy changes however, are additive because G is a state function : ΔG∘3=ΔG∘1+ΔG∘2
Standard electrode potential for the half-cell Fe3+/Fe2+ of the reaction (3) is :
View SolutionRead the following passage for the evaluation of E∘ when different number of electrons are involved. Consider addition of the following half reactions
(1) Fe3+(aq)+3e−→Fe(s)E∘1=0.45V
(2) Fe(s)→Fe2+(aq)+2e−E∘2=−0.04V
(3) Fe3+(aq)+e−→Fe2+(aq)E∘3=?
Because half-reactions (1) and (2) contains a different number of electrons, the net reaction (3) is another half-reaction and E∘3 can't be obtained simply by adding E∘1 and E∘3. The free - energy changes however, are additive because G is a state function : ΔG∘3=ΔG∘1+ΔG∘2
For the reactions
(1) MnO−4+8H++5e−→Mn2+4H2O
E∘=1.51V
(2) MnO2+4H++2e−→Mn2+2H2O
E∘=1.32 then for the reaction
(3) MnO−4+4H++3e−→MnO2+2H2O,E∘ is
View SolutionA hydrogen electrode placed in a buffer solution of CH3COONa and CH3COOH in the ratios of x:y and y:x has electrode potential values E1 volts and E2 volts, respectively at 25∘C The pKa values of acetic acid is (E1 and E2 are oxidation potential)
View SolutionConsider the following E1/SN1 reaction :
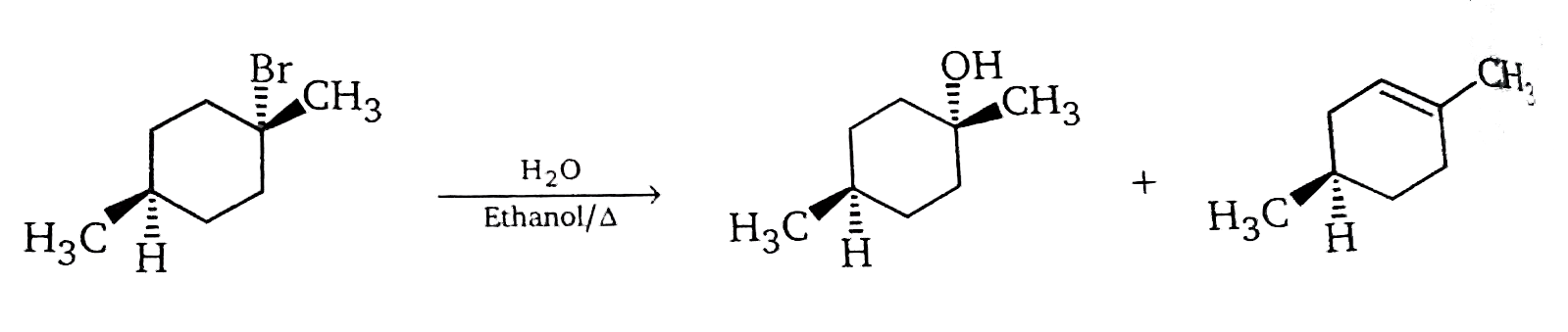
the missing prduct (a) is (are):
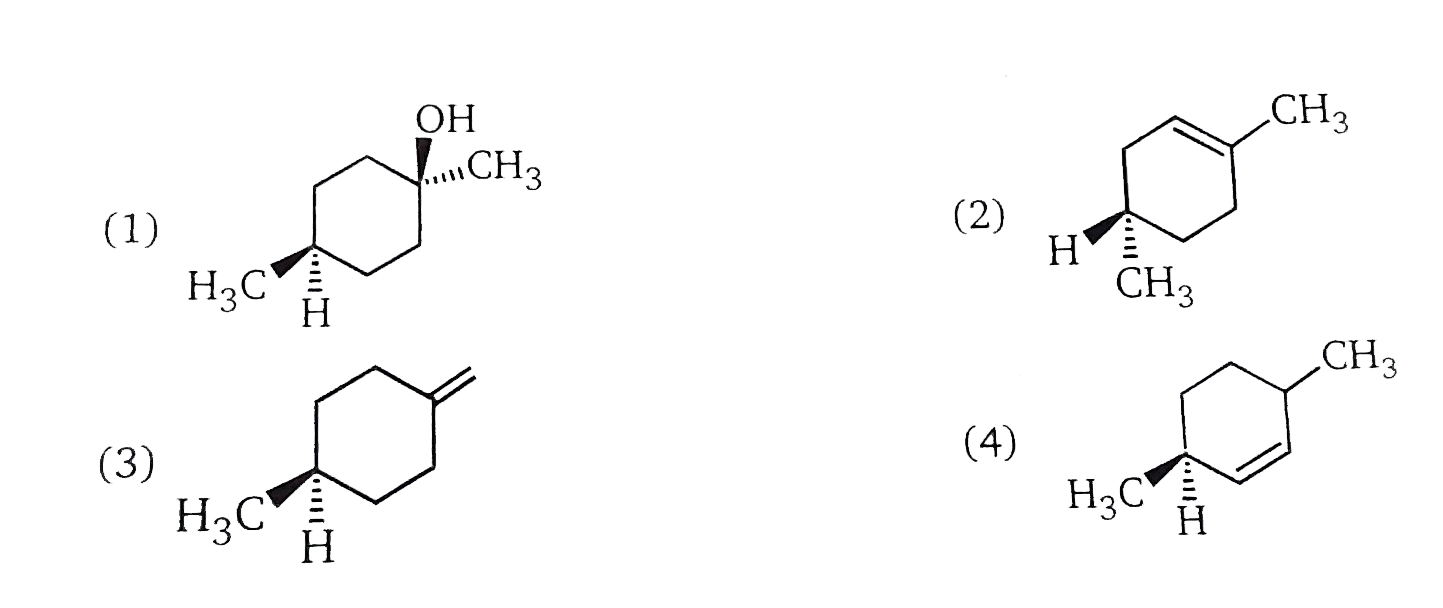
View Solution
