Consider a volatic cell based on these half -cell :
Ag+(aq.)+e−→Ag(s),E∘=+0.80V
Cd2+(aq)+2e−→Cd(s),E∘=−0.40V
Identify the anode and give the voltage of this cell under standard conditions
Ag,Ecell=0.40V
Ag,Ecell=2.00V
Cd,Ecell=1.20V
Cd,Ecell=2.00V
The correct Answer is:C
Anode has lower standard reduction potential, thus Cd will be considered as anode
E∘Cell=ECathode−EAnode
EAg+/Ag−E∘Cd2+/Cd
=0.80−(−0.40)=1.20V
Similar Questions
Al3+(aq)+3e−→Al(s)E∘=−1.66V
Mn2+(aq)+2e−→Mn(s)E∘=−1.18V
What process occurs at the anode of a volatic cell utilizing these two half-reactions?
View SolutionAl3+(aq)+3e−→Al(s) E∘=−1.66V
Cu2+(aq)+2e−→Cu(s) E∘=+0.34V
What voltage is produced under standard conditions by combining the half-reactions witih these standard Electrode Potential?
View Solution
Knowledge Check
Consider a voltaic cell based on these half - cell reactions
Ag+(aq)+e−→Ag(s):E∘=+0.80V
Cd+2(aq)+2e−→Cd(s),E∘=−0.40V
identify the anode and give the voltage of this cell under standard condition.AAg,E∘cell=0.40VBAg,E∘cell=2.00VCCd,E∘cell=1.20VDCd,E∘cell=2.00VAg+(aq)+e−→Ag(s)E∘=0.80V
Cu2+(aq)+2e−→Cu(s)E∘=0.34V
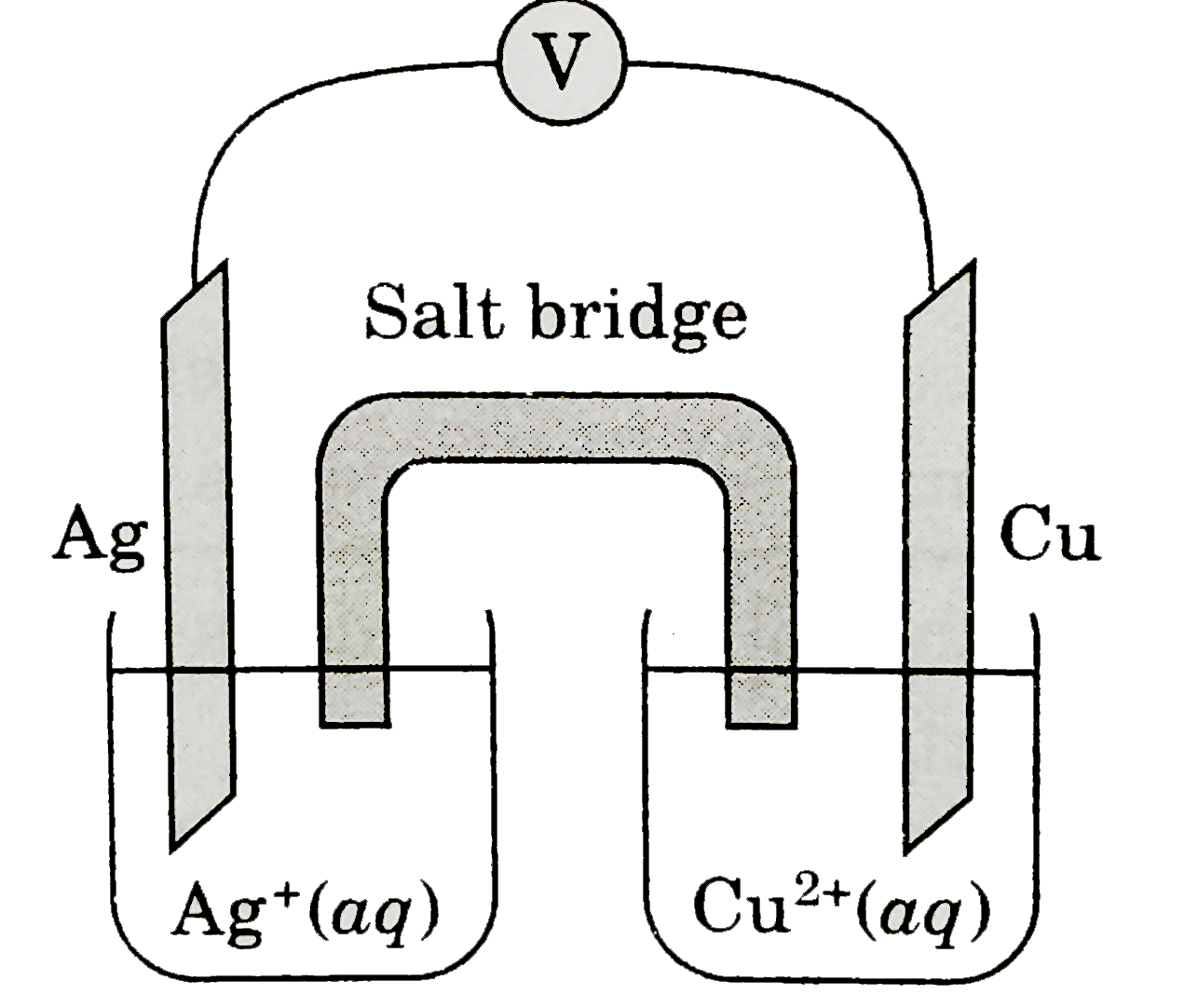
Which expression gives the voltage for this cell if [Cu2+]=1.00M and [Ag+]=0.010M?A0.46V+0.0591VB0.46V+2xx0.0591VC0.46V-0.0591VD0.46V-2xx0.0591VAg+(aq)+e−→Ag(s)E∘=0.80V
Cu2+(aq)+2e−→Cu(s)E∘=0.34V

Which increases immediately if the surface area of the silver electrode is increased?AOverall cell voltageBRate of change of [Ag+]CMass of Cu electrodeDChange in ratio of electrode masses Δ(Mass of CuMass of Ag)
The standard potential E∘ for the half reactions are as :
Zn→Zn2++2e−,E∘=0.76V
Cu→Cu2++2e−,E∘=−0.34V
The standard cell voltage for the cell reaction is ?
Zn+Cu2→Zn2++Cu
View SolutionThe standard reduction potential for two reactions are given below
AgCl(s)+e−→Ag(s)+Cl−(aq),E∘=0.22V
Ag+(aq)+e−→Ag(s),E∘=0.80V
The solubility product of AgCl under standard conditions of temperature is given by
View SolutionThe standard rectuion potentials for two half-cell reactions are given below
Cd2+(aq)+2e−→Cd(s),E∘=−0.40V
Ag+(aq)+e−→Ag(s),E∘=0.80V
The standard free energy change for the reaction
2Ag+(aq)+Cd(s)→2Ag(s)+Cd2+(aq) is given by
View SolutionThe standard electrode potentials for the ractions
Ag+(aq)+e−→Ag(s)
sn+(aq)+2e−→Sn(s)
at 25∘C are 0.80 V and -0.14 V respectively the emf of the cell Sn∣∣Sn2+∣∣(1M)∣∣Ag+(1M)∣∣ Ag is
View SolutionThe standard electrode potential for the reactions,
Ag+(aq)+e−→Ag(s)
Sn2+(aq)+2e−→Sn(s)
at 25∘C are 0.80 volt and −0.14 volt, respectively. The emfof the cell Sn∣∣Sn2+(1M)∣∣∣Ag+(1M)Ag is :
View Solution

