36.4 g of 1,1,2,2-tetrachoropropane was heated with zinc dust and the product was bubbled through ammoniacal AgNO3. What is the mass of precipitatee obtained? Give equation for the reaction involved.
Text Solution
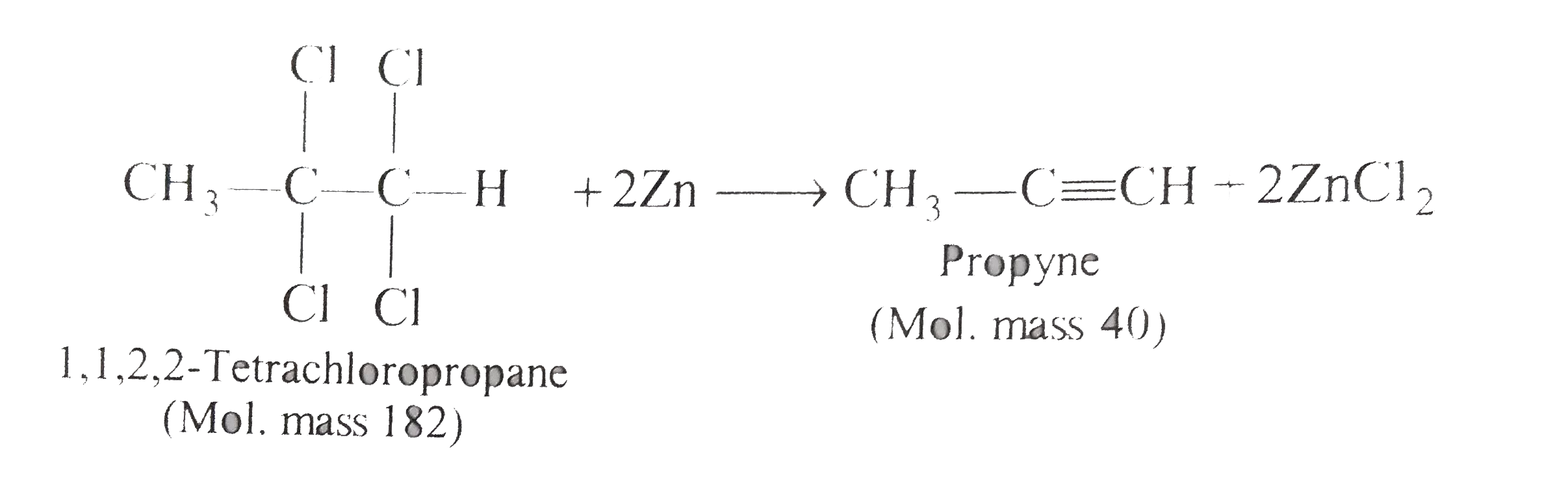
Mass of propyne obtained from 36.4 g of
1,1,2,2-tetrachloropropane =40182×36.4=8g
CH3−C=−CH+AgNO3→CH3−C=−C−Ag+NH4NO3+H2O
Mass of precipate obtained from 8g of propyne
=14740×8=29.4 g
|
Similar Questions
Knowledge Check
1,1,2,2- tetrachloropropane was heated with zinc dust and the product was bubbled through ammonical AgNO3. What is the weight of precipitate obtained?
A30.0gB29.4gC28.0gD25.7g1,3-Diiodopropane is heated with zinc dust in ether. The product formed is
A3-bromopropaneBpropaneCpropeneDcyclopropane1,3 -Dibromo propane if heated with zinc dust in ether ,the product formed is:
APropeneBPropaneCCyclopropaneD3-Bromopropane
If 1,3–dibromopropane reacts with zinc dust, the product obtained is:
View Solution1, 2 - dibromopropane , when heated with Zn dust in ethanol, gives
View Solution2-bromopentane is heated with postassium ethoxide in ethano1 The major product obtained is .
View SolutionSample of 2,3-dibromo-3-methylpentane is heated with zinc dust. The resulting products is isolated and heated with HI in the presence of phosphorus. Indicate which is the structure that represents the final product in the reaction:
View SolutionWhen 3,3−dimethyl−2−butanol is heated with H2SO4 the major product obtained is
View Solution

