Write the structural and stereoisomers of pentene, C5H10 and compare their stabilities.
Video Solution
Text Solution
Pentene exists in the following isomeric forms:
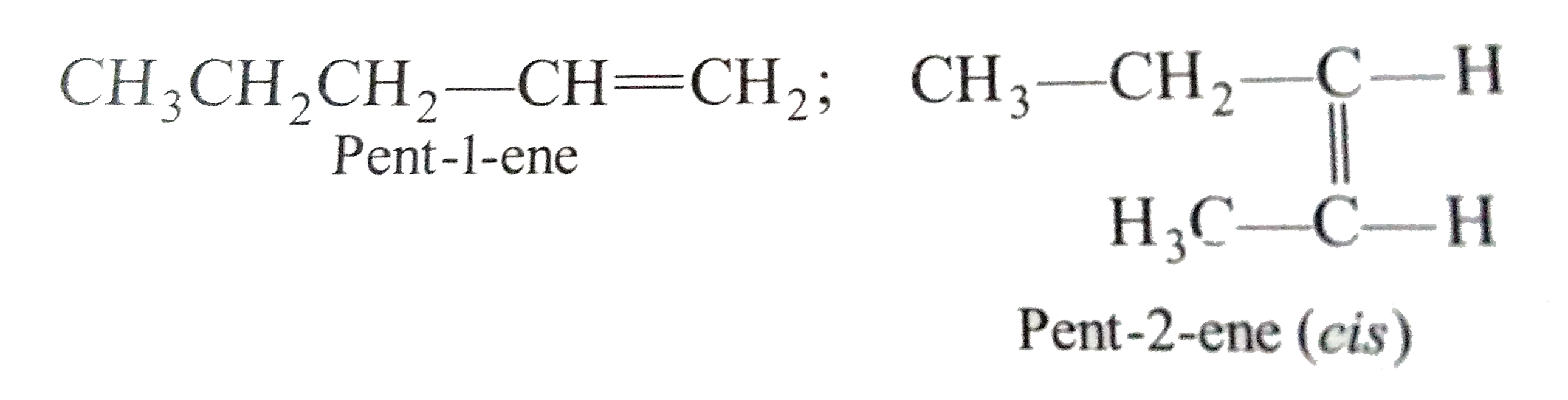
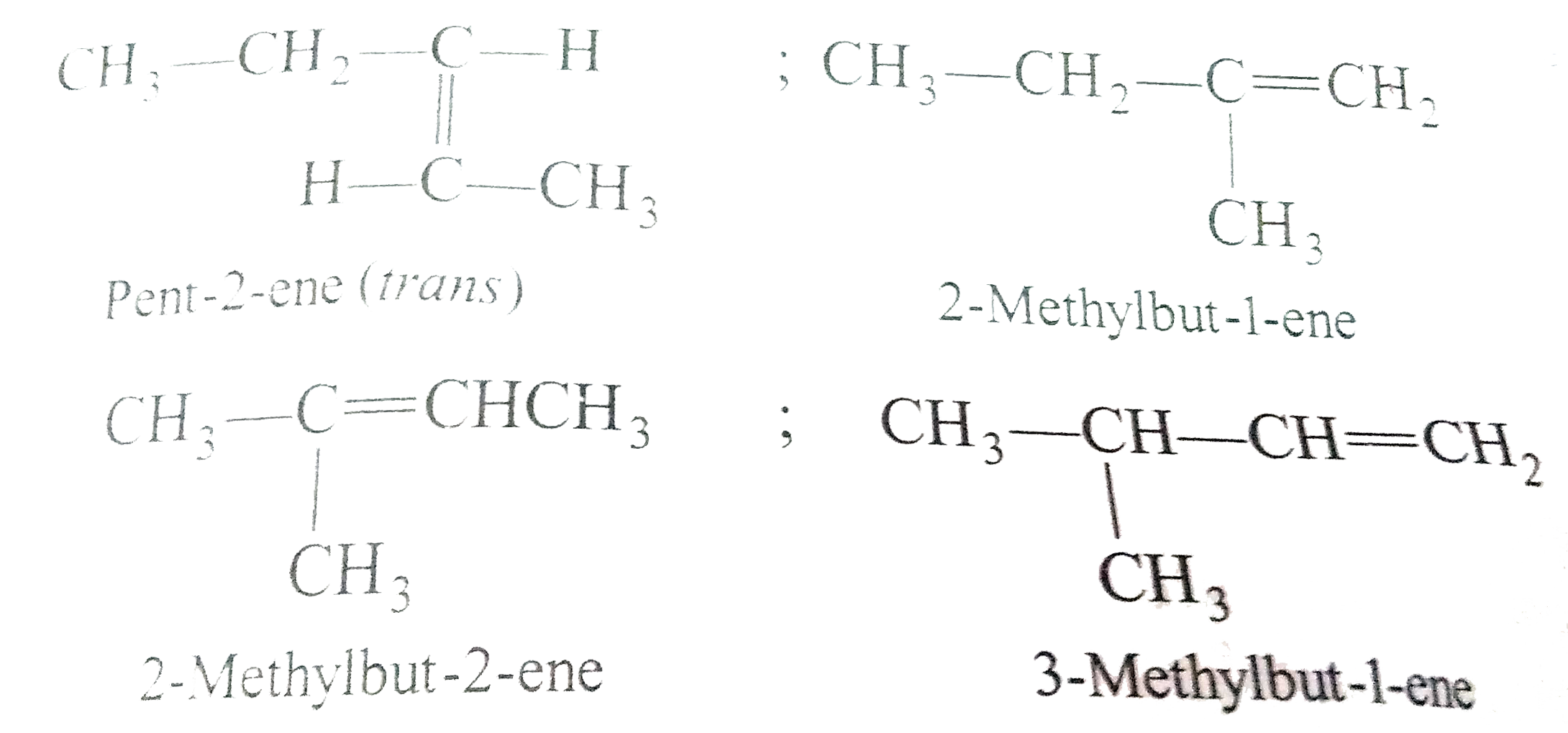
and the stability order is:
Pent-1-ene < pent-2-ene(cis) < pent-2-ene(trans) <2-methylbut-2-ene
(Sinc, stability increases with increasing number of electron releasing alkyl groups.
|
Similar Questions
Knowledge Check
The number of structural isomers for C5H10 are:
A8B6C9D10The number of isomers (including stereoisomers) of C5H10 are
A10B11C12D13How many cyclic structural isomer of C5H10 are possible
A5B3C4D6
Write the structural formulae and IUPAC names of the isomers represented by C5H12.
View SolutionDraw the structures of six isomeric pentenes, C5H10 Specify as E or Z to each geometric isomer.
View Solutiona) Draw the stereochemical structure of the product in the following reaction.
R−C≡C−RH2−−−−−−−−−→Lindlar's catalyst
b) Write down the structures of the stereoisomers formed when cis-2-butene is reacted with bromine.
View SolutionHow many cyclic structural isomers are possible for C5H10?
View SolutionThe number of structural isomers (open chain) possible for C5H10 are
View Solution
