One per cent composition of an organic compound A is carbon=85.71% and hydrogen =14.29%. Its vapour density is 14. Consider the following reaction sequences.
ACl2/H2O−−−−−→(B)KCN/EtOH−−−−−−−→H3O+C, Identify C.
CH3−C∣OHO2H
HO−CH2−CH2−CO2H
HO−CH2−CO2H
CH3CH2−CO2H
The correct Answer is:B
Step by step video, text & image solution for One per cent composition of an organic compound A is carbon=85.71% and hydrogen =14.29%. Its vapour density is 14. Consider the following reaction sequences. A overset(Cl_(2)//H_(2)O)to(B) overset(KCN//EtOH)underset(H_(3)O^(+))toC, Identify C. by Chemistry experts to help you in doubts & scoring excellent marks in Class 12 exams.
Updated on:21/07/2023Similar Questions
Identify A,B and C in the following reactions
CH3ClKCN−−→Ahydrolysis−−−−−→H3O⊕BC2H5OH/H+−−−−−−−−→ΔC
View SolutionIdentify A,B and C in the following reactions CH_(3)Cl overset(KCN) to A underset(H_(3)O^(o+))overset("hydrolysis")toB underset(Delta)overset(C_(2)H_(5)OH//H^(+))toC
View SolutionIdentify A, B, C , -------- in the following. EtOH overset(PCl_(5))to A underset(Et_(2)O)overset(Mg)toBunderset(2. H_(3)O^(+))overset(1."cyclohexanone")toC
View SolutionC_(2)underset((A))(H_(4))Cl_(2)overset(KCN)to(B)overset(H_(2)O^(+))to(C)overset(Delta)to(D) The compound D has the characteristic(s)
View SolutionIn the following sequence of reactions, the compound C formed would be
CH3−CH| NH2−CH3HNO2−−−→H2OA[O]−−→BCH3MgI−−−−−→H+/H2OC
View SolutionThe percent composition of an organic compound A–– is carbon : 85.71 % and hydrogen 14.29%. Its vapour density is 14. Consider the following reaction sequence :
A−Cl2/H2O−−−−−→B−(1)KCN/EtOH−−−−−−−−−−→(2)H3O+C− Identify C−
View SolutionIdentify the compound C in the following sequence:
(CH3)2CHCH2C≡NHCl,H2O−−−−−→compound A1.LiAlH4−−−−−→2.H2Ocompound BPC−−−−→CH2Cl2Compound C
View SolutionIdentify A to G in the following.
(a)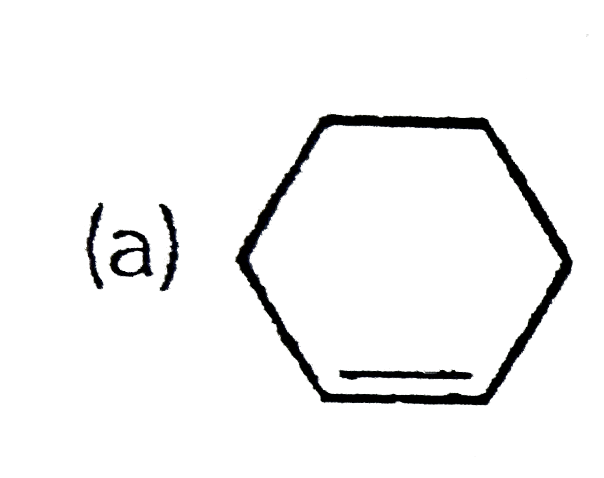 Br2,CCl4−−−−−→AKCN−−→BH3O+−−−→C
Br2,CCl4−−−−−→AKCN−−→BH3O+−−−→C
(b)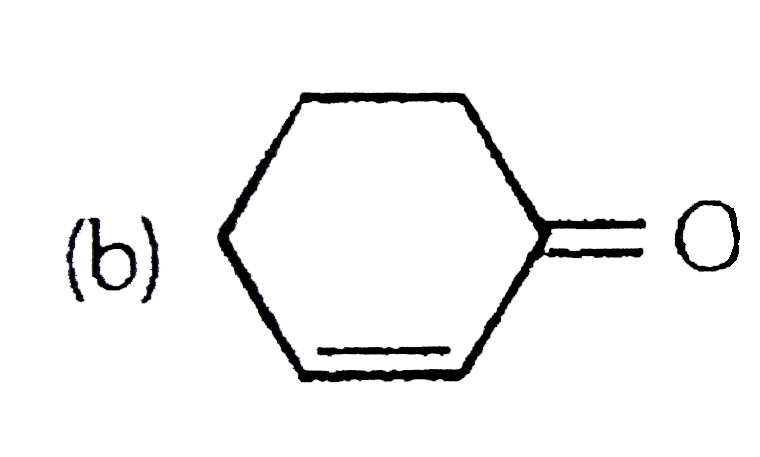 Br2,CCl4−−−−−→DKCN−−→EH3O+−−−→G
Br2,CCl4−−−−−→DKCN−−→EH3O+−−−→G
View Solution
