A) Acidity of C-H bond lies in following sequences
HC≡CH>CH2=CH2>CH3−CH3
R) Percentage character of 's' orbital in these compounds lie in following sequences.
H−C≡CH>CH2=CH2>CH3−CH3
If both (A) and ( R) are correct and (R ) is correct explanation of (A).
If both (A) and (R ) are correct and (R ) is not correct explanation of (A).
If (A) is correct but ( R) is correct.
If (A) is incorrect but (R ) is correct.
Step by step video & image solution for A) Acidity of C-H bond lies in following sequences HC-=CH gt CH_(2) = CH_(2) gt CH_(3)-CH_(3) R) Percentage character of 's' orbital in these compounds lie in following sequences. H-C-=CH gt CH_(2) = CH_(2) gt CH_(3)-CH_(3) by Chemistry experts to help you in doubts & scoring excellent marks in Class 12 exams.
Updated on:21/07/2023Topper's Solved these Questions
- UNSATURATED HYDROCARBONSBook:OP TANDONChapter:UNSATURATED HYDROCARBONSExercise:MATRIX MATCH TYPE QUESTIONS:
Explore 6 Videos
- UNSATURATED HYDROCARBONSBook:OP TANDONChapter:UNSATURATED HYDROCARBONSExercise:LINKED COMPREHENSION TYPE QUESTIONS
Explore 21 Videos
- UNSATURATED HYDROCARBONSBook:OP TANDONChapter:UNSATURATED HYDROCARBONSExercise:SET II This set contains the questions with more ..
Explore 23 Videos
- STOICHIOMETRY (CHEMICAL FORMULAE AND EQUATIONS )Book:OP TANDONChapter:STOICHIOMETRY (CHEMICAL FORMULAE AND EQUATIONS )Exercise:SELF ASSESSMENT
Explore 11 Videos
Similar Questions
Knowledge Check
In the compound HC≡C−CH2−CH=CH−CH3, the C2−C3 bond is the type of :-
Asp−sp2Bsp3−sp3Csp−sp3Dsp2−sp2What hybrid orbitals will form the following compound H3C−CH=CH−CH2−CH3
Asp and sp3Bsp2 and sp3Csp and sp3DOnly sp3Arrange the following compounds in decreasing order of acidity :
CH3CH2OH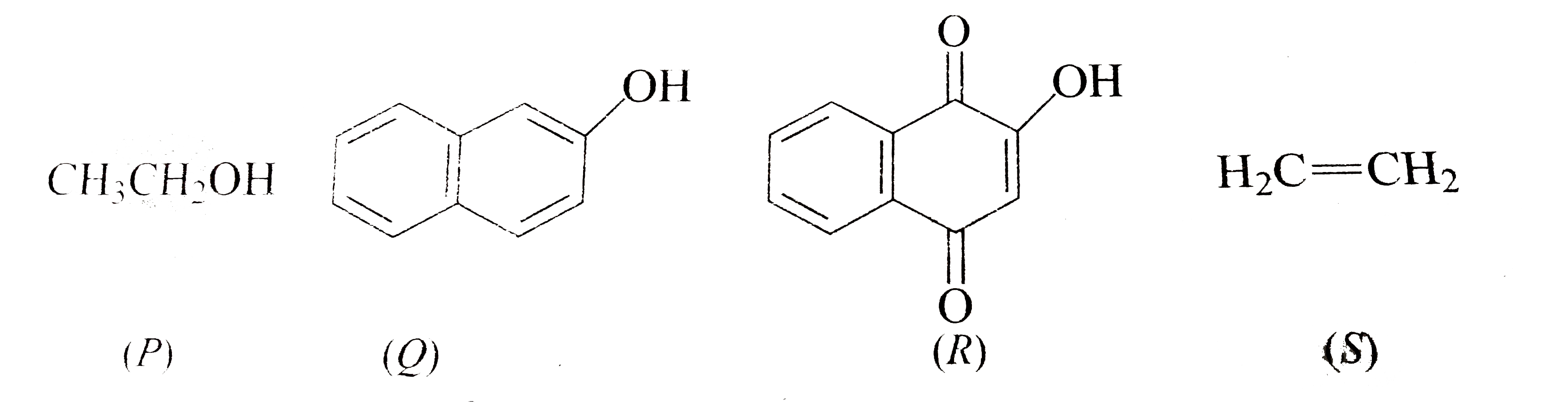
H2C=CH2AR>Q>P>SBQ>R>P>SCS>R>Q>PDQ>P>R>S
What is the kind of isomerism exhibited by the compounds given below?
CH3−C≡C−CH2−CH2−CH3CH3−CH2−C≡C−CH2−CH3
View SolutionIn the compound
CH2=CH−CH2−CH2−C≡CH the C2−C3 bond is of
View SolutionIUPAC name of the following compound will be
CH3−CH=C−CH2−CH3 | CH2−CH2−CH3
View SolutionIUPAC name of the following compound will be
CH3−CH=C−CH2−CH3 | CH2−CH2−CH3
View SolutionS-I: Acidic character of ethane, ethene and ethyne are in the order
CH≡CH>CH2=CH2>CH3−CH3
S-II: Higher the s-character in hybridization of carbon atom, more is the acidic nature of H atom joined to it.
View Solution
