Hydrogenation of alkenes and alkynes takes place in presence of certain catalysts. In Sabatier Senderen's reaction, the addition of hydrogen takes place I presence of Raney nickel catalyst. Platininm and palladium can also be used as catalyst and used in finley divided state. Experimentally, it is observed that less crowded alkenes adsorb H2 with faster rate. Controlled hydrogenation of alkyne H2 with faster rate. Controlled hydrogenationof alkyne in presence of Lindlar's catalyst yields cis product i.e., 'cis' alkene. Thus, in presence of Lindlar's catalyst 'syn' addition takes place. The relative rate of hydrogenation follows the order:
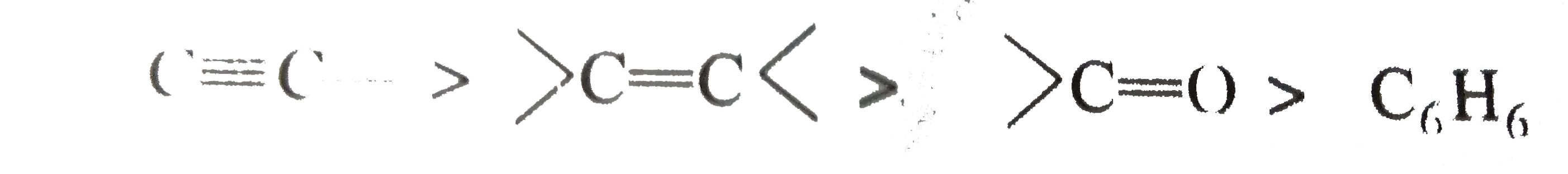
Non-terminal alkynes are reduced in presence of Na or Li metal dissolved in liquid ammonia. In this reaction, anti-addition of hydrogen results into the trans-product.
The product of the following reaction is:
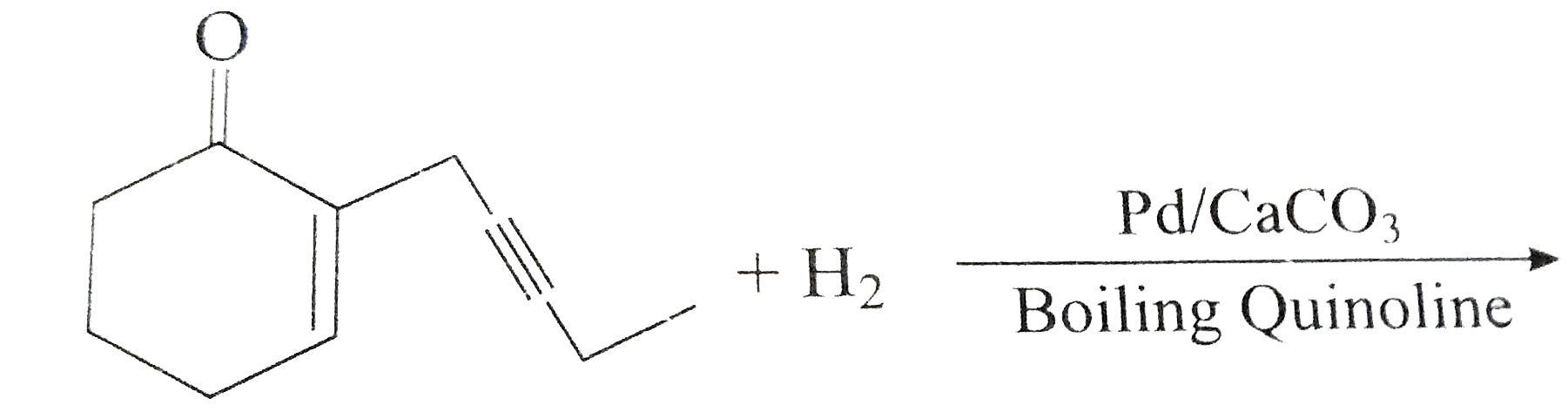
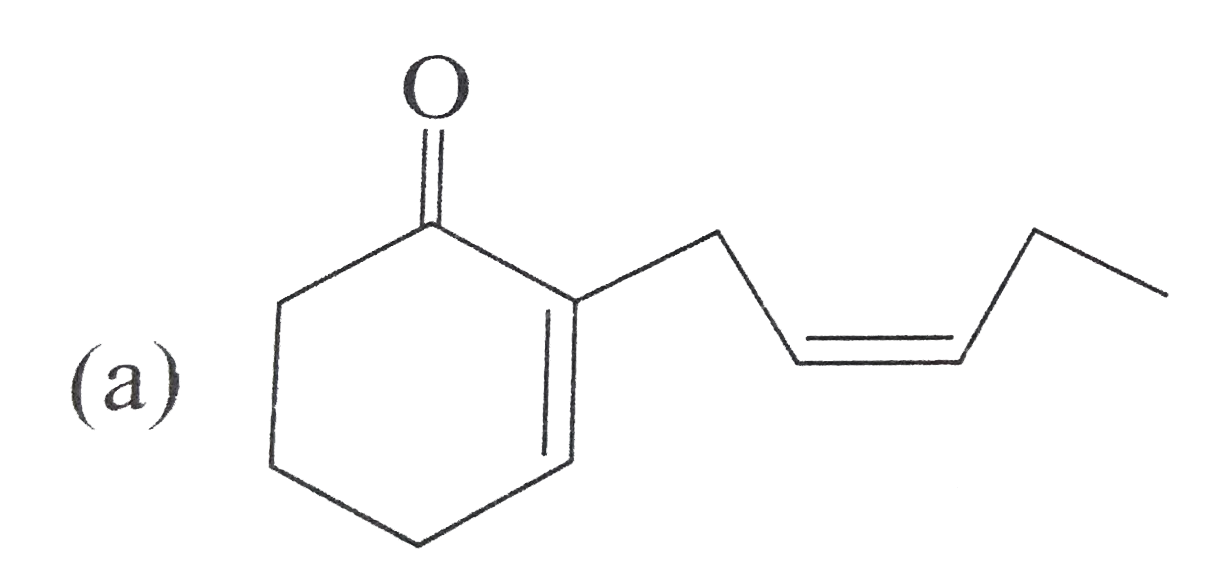
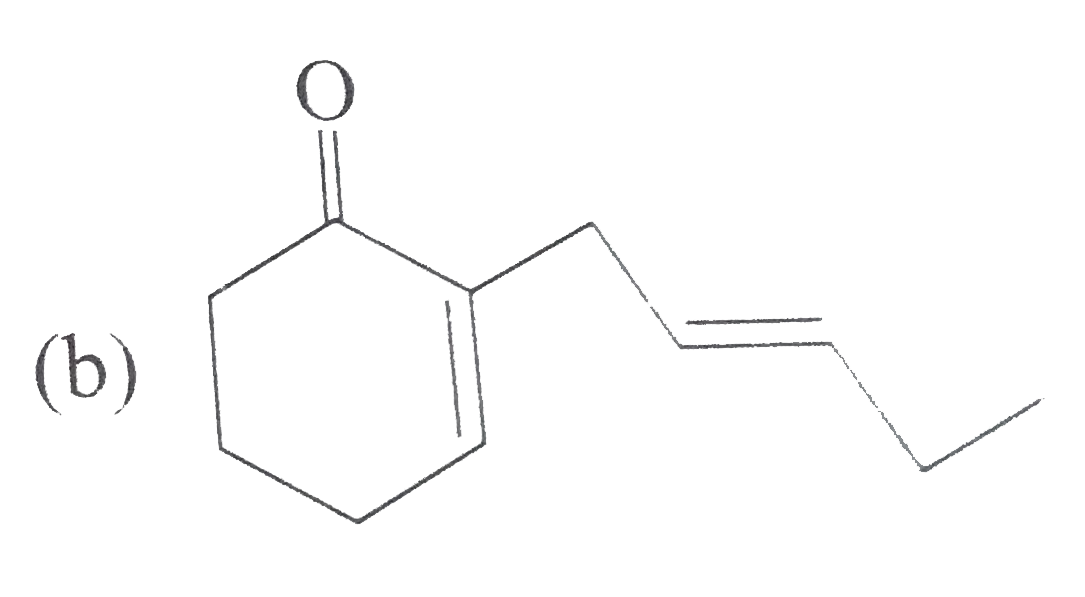
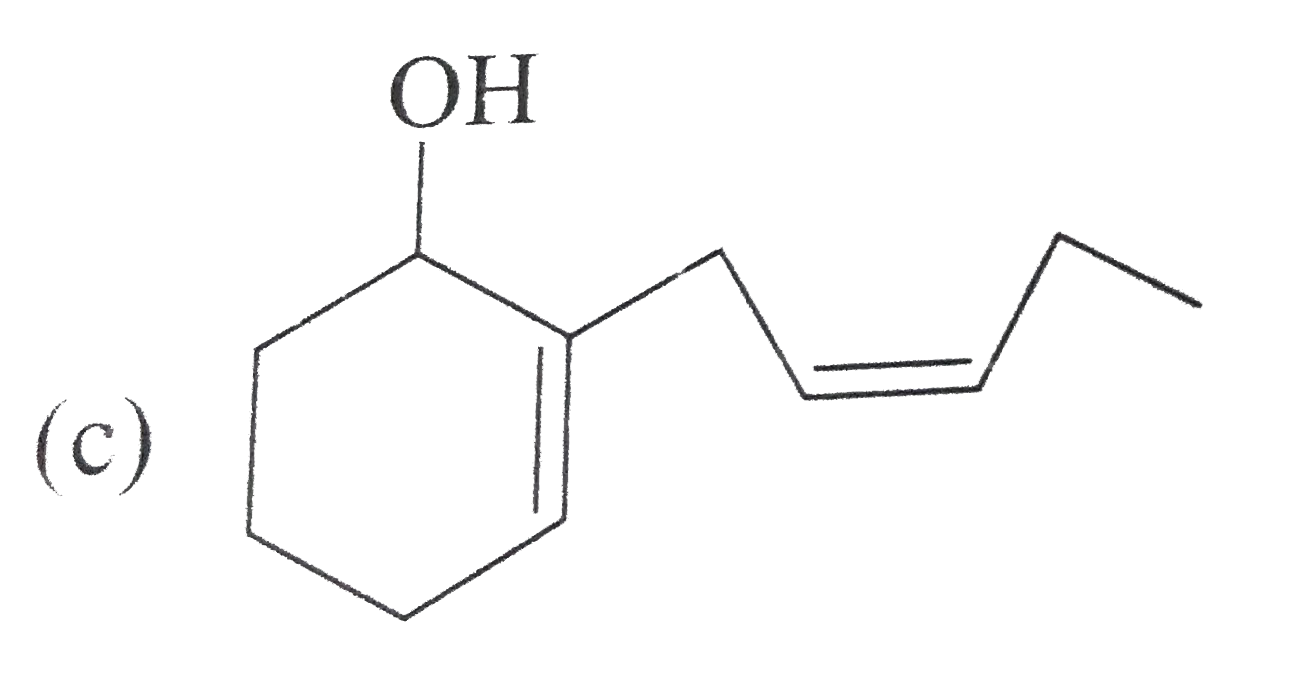
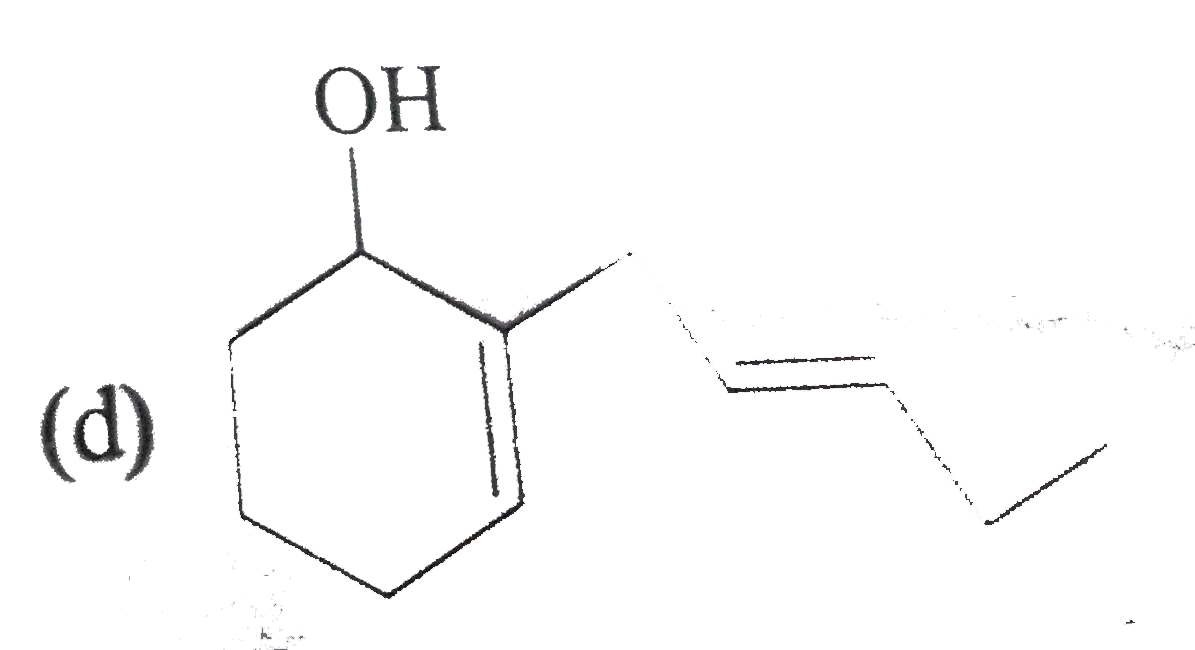
The correct Answer is:A
Step by step video, text & image solution for Hydrogenation of alkenes and alkynes takes place in presence of certain catalysts. In Sabatier Senderen's reaction, the addition of hydrogen takes place I presence of Raney nickel catalyst. Platininm and palladium can also be used as catalyst and used in finley divided state. Experimentally, it is observed that less crowded alkenes adsorb H_(2) with faster rate. Controlled hydrogenation of alkyne H_(2) with faster rate. Controlled hydrogenationof alkyne in presence of Lindlar's catalyst yields cis product i.e., 'cis' alkene. Thus, in presence of Lindlar's catalyst 'syn' addition takes place. The relative rate of hydrogenation follows the order: Non-terminal alkynes are reduced in presence of Na or Li metal dissolved in liquid ammonia. In this reaction, anti-addition of hydrogen results into the trans-product. The product of the following reaction is: by Chemistry experts to help you in doubts & scoring excellent marks in Class 12 exams.
Updated on:21/07/2023Topper's Solved these Questions
- UNSATURATED HYDROCARBONSBOOK - OP TANDONCHAPTER - UNSATURATED HYDROCARBONSEXERCISE - SINGLE INTEGER ANSWER TYPE QUESTIONS
10 Videos
- UNSATURATED HYDROCARBONSBOOK - OP TANDONCHAPTER - UNSATURATED HYDROCARBONSEXERCISE - MATRIX MATCH TYPE QUESTIONS:
6 Videos
- STOICHIOMETRY (CHEMICAL FORMULAE AND EQUATIONS )BOOK - OP TANDONCHAPTER - STOICHIOMETRY (CHEMICAL FORMULAE AND EQUATIONS )EXERCISE - SELF ASSESSMENT
11 Videos
Similar Questions
If a mixture containing 1-butene, cis-2-butene and trans-2-butene is treated with perbenzoic acid, how many different isomers of epoxides result?
View SolutionMatch the following:
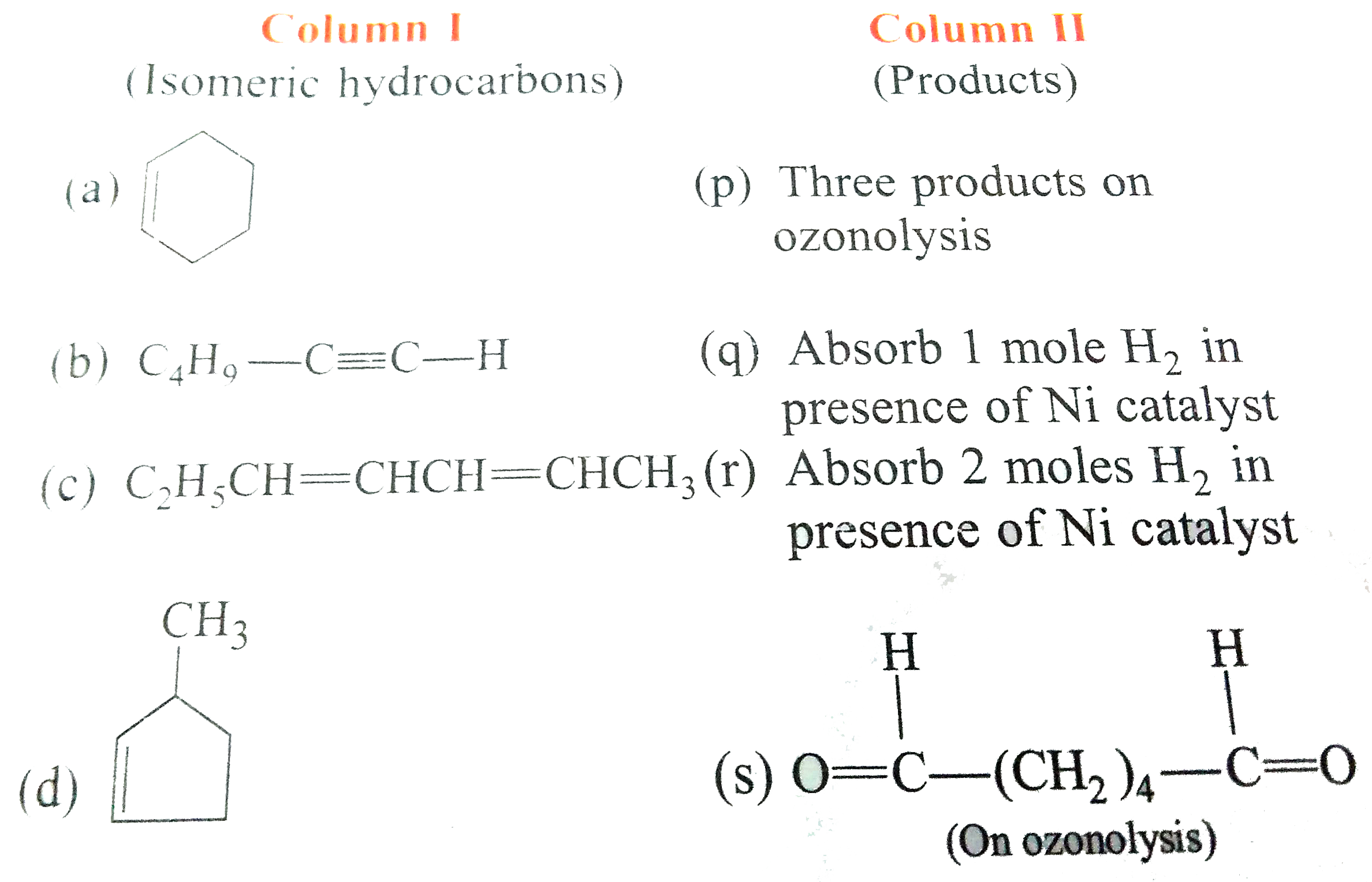
View SolutionAn oxide of iodine contains 25.4g of iodine for 8g of oxygen. Its molecular formula will be:
View SolutionAlkyne can be reduced to alkenes by hydrogenation in presence of
View SolutionHydrogenation of alkenes and alkynes takes place in presence of certain catalysts. In sabatier-Senderen's reaction, the addition of hydrongen taken place in presence of nickel catalyst. Controlled hydrogenation of alkyne in presence of Lindlar's catalyst gives cis-alkene. Non terminal alkynes are reduced by Na or Li metal dissolved in liquid NH3. In this reaction trans alkenes are formed.
Q. In which of the following the reaction is most exothermic
View SolutionHydrogenation of alkenes and alkynes takes place in presence of certain catalysts. In sabatier-Senderen's reaction, the addition of hydrongen taken place in presence of nickel catalyst. Controlled hydrogenation of alkyne in presence of Lindlar's catalyst gives cis-alkene. Non terminal alkynes are reduced by Na or Li metal dissolved in liquid NH3. In this reaction trans alkenes are formed.
Q. The product of the following reaction is
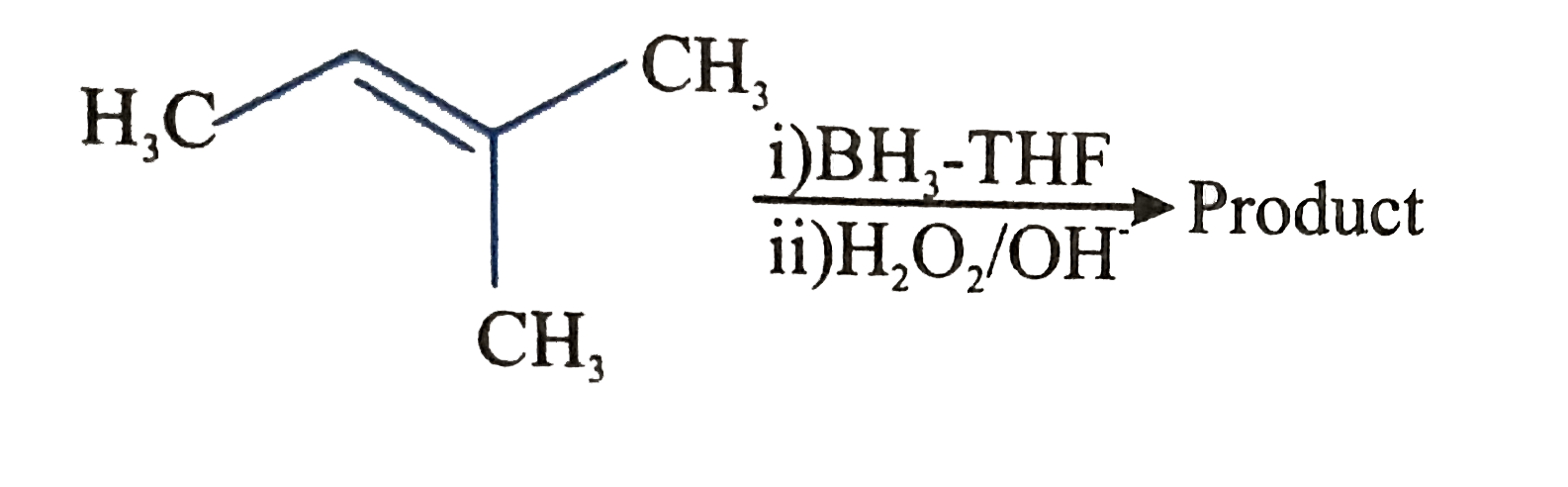
View SolutionHydrogenation of alkenes and alkynes takes place in presence of certain catalysts. In Sabatier Senderen's reaction, the addition of hydrogen takes place I presence of Raney nickel catalyst. Platininm and palladium can also be used as catalyst and used in finley divided state. Experimentally, it is observed that less crowded alkenes adsorb H2 with faster rate. Controlled hydrogenation of alkyne H2 with faster rate. Controlled hydrogenation of alkyne in presence of Lindlar's catalyst yields cis product i.e., 'cis' alkene. Thus, in presence of Lindlar's catalyst 'syn' addition takes place. The relative rate of hydrogenation follows the order:
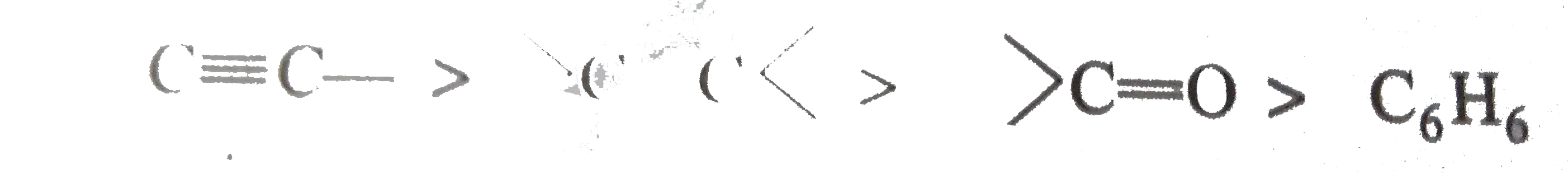
Non-terminal alkynes are reduced in presence of Na or Li metal dissolved in liquid ammonia. In this reaction, anti-addition of hydrogen results into the trans-product.
Answer the following questions:
The relative rate of catalytic hydrogenation of following alkenes is:
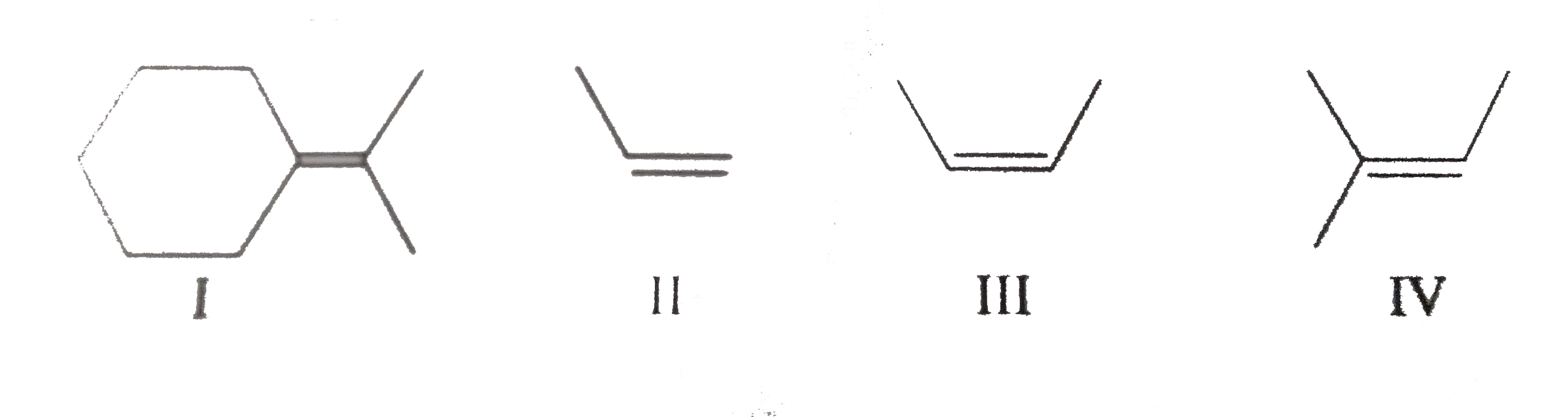
View SolutionHydrogenation of alkenes and alkynes takes place in presence of certain catalysts. In Sabatier Senderen's reaction, the addition of hydrogen takes place I presence of Raney nickel catalyst. Platininm and palladium can also be used as catalyst and used in finley divided state. Experimentally, it is observed that less crowded alkenes adsorb H2 with faster rate. Controlled hydrogenation of alkyne H2 with faster rate. Controlled hydrogenation of alkyne in presence of Lindlar's catalyst yields cis product i.e., 'cis' alkene. Thus, in presence of Lindlar's catalyst 'syn' addition takes place. The relative rate of hydrogenation follows the order:
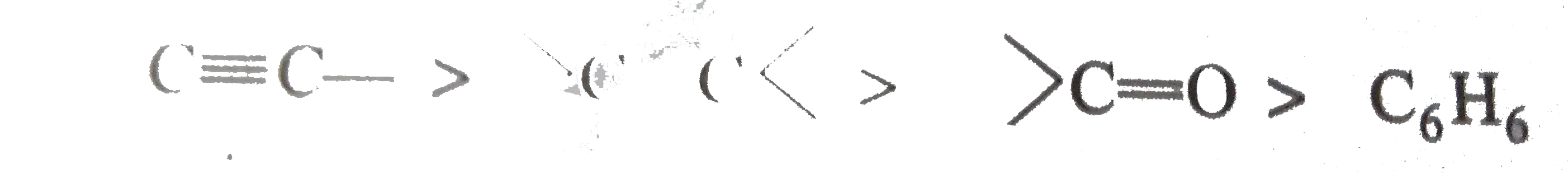
Non-terminal alkynes are reduced in presence of Na or Li metal dissolved in liquid ammonia. In this reaction, anti-addition of hydrogen results into the trans-product.
Answer the following questions:
The relative rate of catalytic hydrogenation of following alkenes is:
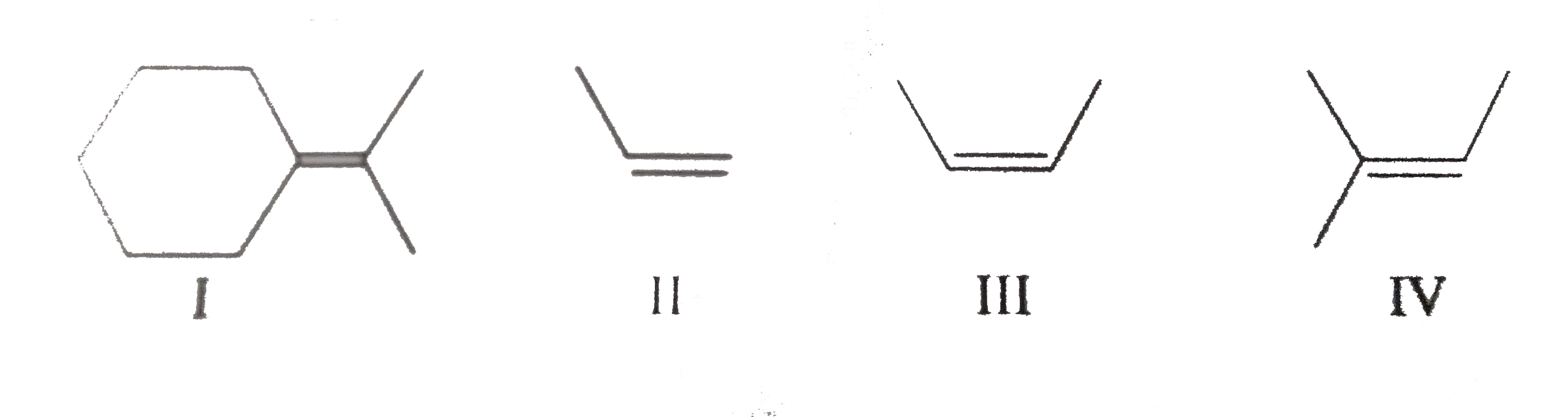
View SolutionHydrogenation of alkenes and alkynes takes place in presence of certain catalysts. In Sabatier Senderen's reaction, the addition of hydrogen takes place I presence of Raney nickel catalyst. Platininm and palladium can also be used as catalyst and used in finley divided state. Experimentally, it is observed that less crowded alkenes adsorb H2 with faster rate. Controlled hydrogenation of alkyne H2 with faster rate. Controlled hydrogenation of alkyne in presence of Lindlar's catalyst yields cis product i.e., 'cis' alkene. Thus, in presence of Lindlar's catalyst 'syn' addition takes place. The relative rate of hydrogenation follows the order:
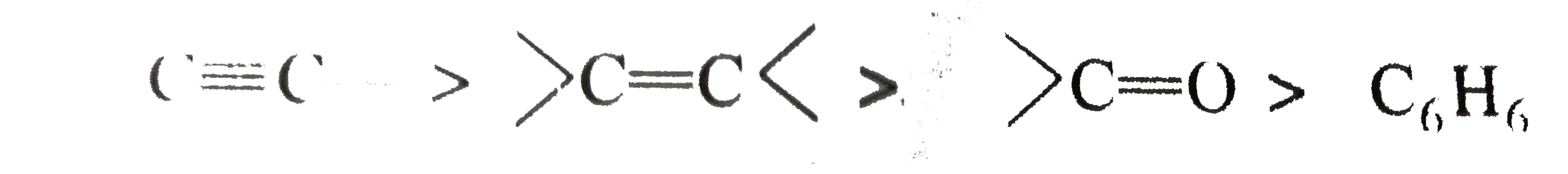
Non-terminal alkynes are reduced in presence of Na or Li metal dissolved in liquid ammonia. In this reaction, anti-addition of hydrogen results into the trans-product.
H3C−C≡C−CH3+H2Pd/CaCO3−−−−−−−−−→Boiling Quinoline(A)
The product (A) will be:
View SolutionHydrogenation of alkenes and alkynes takes place in presence of certain catalysts. In Sabatier Senderen's reaction, the addition of hydrogen takes place I presence of Raney nickel catalyst. Platininm and palladium can also be used as catalyst and used in finley divided state. Experimentally, it is observed that less crowded alkenes adsorb H2 with faster rate. Controlled hydrogenation of alkyne H2 with faster rate. Controlled hydrogenation of alkyne in presence of Lindlar's catalyst yields cis product i.e., 'cis' alkene. Thus, in presence of Lindlar's catalyst 'syn' addition takes place. The relative rate of hydrogenation follows the order:
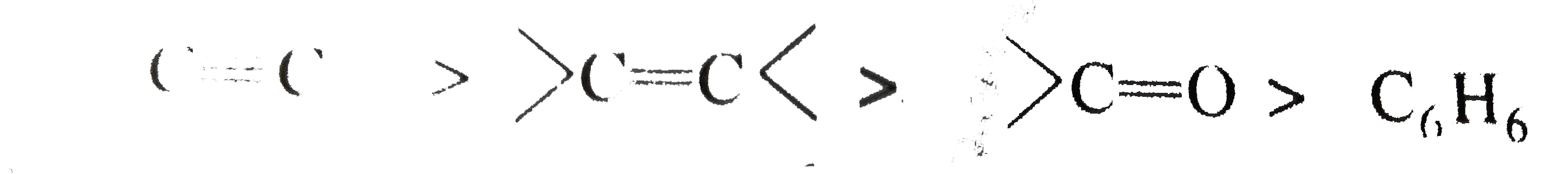
Non-terminal alkynes are reduced in presence of Na or Li metal dissolved in liquid ammonia. In this reaction, anti-addition of hydrogen results into the trans-product.
In which of the following cases, the reaction is most exothermic?
View SolutionHydrogenation of alkenes and alkynes takes place in presence of certain catalysts. In Sabatier Senderen's reaction, the addition of hydrogen takes place I presence of Raney nickel catalyst. Platininm and palladium can also be used as catalyst and used in finley divided state. Experimentally, it is observed that less crowded alkenes adsorb H_(2) with faster rate. Controlled hydrogenation of alkyne H_(2) with faster rate. Controlled hydrognetation of alkyne in presence of Lindlar's catalyst yields cis product i.e., 'cis' alkene. Thus, in presence of Lindlar's catalyst 'syn' addition takes place. The relative rate of hydrogenation follows the order: Non-terminal alkynes are reduced in presence of Na or Li metal dissolved in liquid ammonia. In this reaction, anti-addition of hydrogen results into the trans-product. Powdered nickel is more effective than grannular nickel because:
View SolutionFor selective hydrogenation of alkynes into alkene the catalyst used is
View SolutionFor selective hydrogenation of alkynes into alkene the catalyst used is
View SolutionFor selective hydrogenation of alkynes into alkene the catalyst used is
View SolutionFor selective hydrogenation of alkynes into alkene the catalyst used is
View Solution

