From the following data for the reaction between A and B
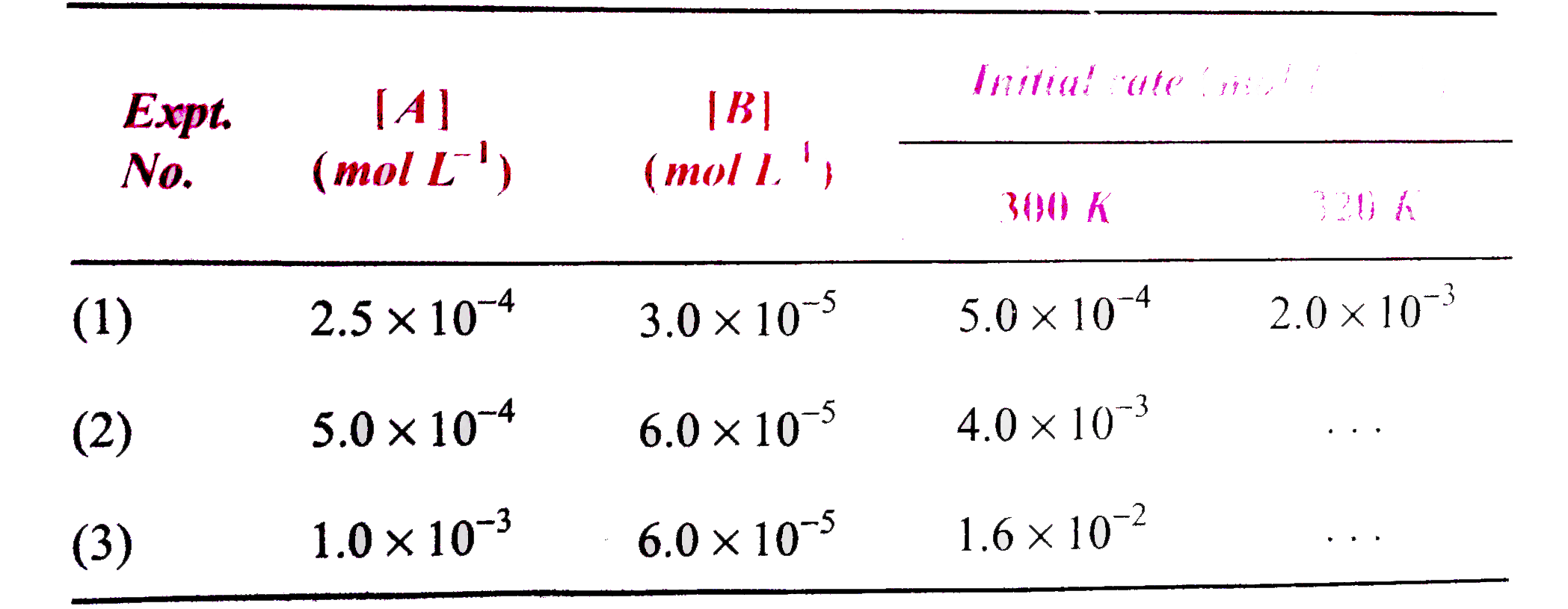
Calculate the following
(i) The order of the reaction with respect to A and with respect to B
(ii) The rate constant at 300K
(iii) The energy of activation and
(iv) The pre-exponential factor.
(i) Let the rat law be :
Rate= k[A]x[B]y
From expt.(1),5.0×10−4=k[2.5×10−4]x[3.0×10−5]y ....(i)
From expt.(2),4.0×10−4=k[5.0×10−4]x[6.0×10−5]y ....(ii)
Dividing by eq. (i), 4.0×10−35.0×10−4=2x⋅2y=8
From expt.(3),1.6×10−2=k[1.0×10−3]x[6.0×10−5]y ....(iii)
From expt.(1),5×10−4=k[2.5×10−4]2[3.0×10−5]
or k=5×10−4[2.5×10−4]2[3.0×10−5]=2.67×108L2mol−2s−1
(iii) From Arrhenius eqauation,
log10.2.0×10−35.0×104=Ea2.3030×8.314×20300×320
Ea=2.303×8.314×300×32020×log104
(iv) Applying log10k=log10A−Ea2.3030RT
log10.Ak=553332.3030×8.314×300=9.633
or Ak=4.29×109
or A=4.29×109×2.67×108
=1.145×1018
Similar Questions
From the following data for the reaction between A and B
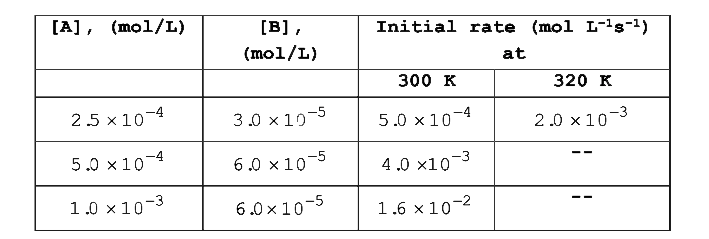
Calculate:
(i) the order of the reaction with respect to A and with respect to B
(ii) the rate constant at 300 K
(iii) the pre-exponential factor.
View Solutionform the following data for the reaction between A and B,

(a) Calculate the order of the reaction with respect to A and with respect to B.
(b) Calculate the rate constant at 300K.
( c) Calculate the pre-expontential factor.
View Solution
Knowledge Check
The rate of reaction between two A and B decreases by factor 4 if the concentration of reactant B is doubled. The order of this reaction with respect to B is
A−1B−2C2D1The rate of reaction between A and B increases by a factor of 100, when the concentration with respect to A is increased 10 folds, the order of reaction w.r.t. A is
A10B1C4D2The rate constant of a reaction will be equal to the pre-exponential factor when
ATemperature in centigrade is zero.BThe absolute temperature is zero.CThe absolute temperature is infinity.DNo suitable answer.
Consider the following data for the reaction:
A+B→Products

Determine the order of reaction with respect to A and with respect to B and the overall order of the reaction.
View SolutionCalculate the order of reaction A+B→P, with respect to A from the following data :

View SolutionThe following data wer obtained for a given reaction at 300 K

Calculate by what factor the rate of catalyst reaction is increases ?
View SolutionFor the raction A+B→ products, it is found that order of A is 2 and the order of B is 3. In the rate expression when the concentration of both A and B are doubled the rate will increases by a factor
View SolutionThe rate of reaction between A and B increases by a factor of 100, when the concentration with respect to A is increased 10 folds, the order of reaction w.r.t. A is
View Solution
