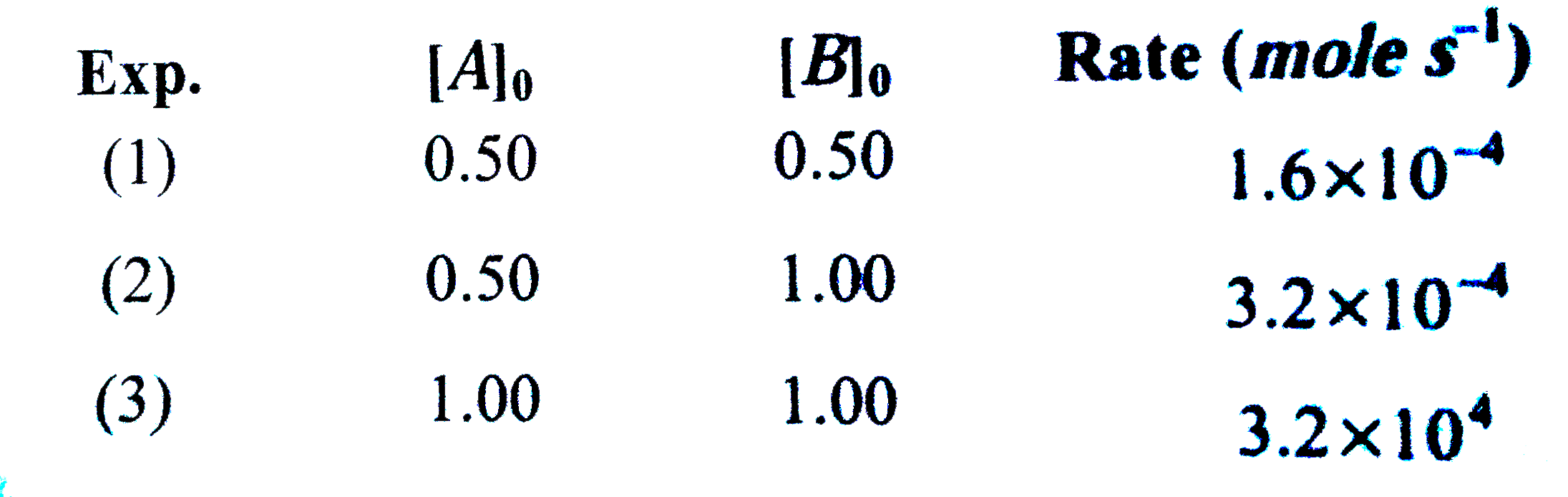The experimental data for the reaction 2A+B2→2AB, is :
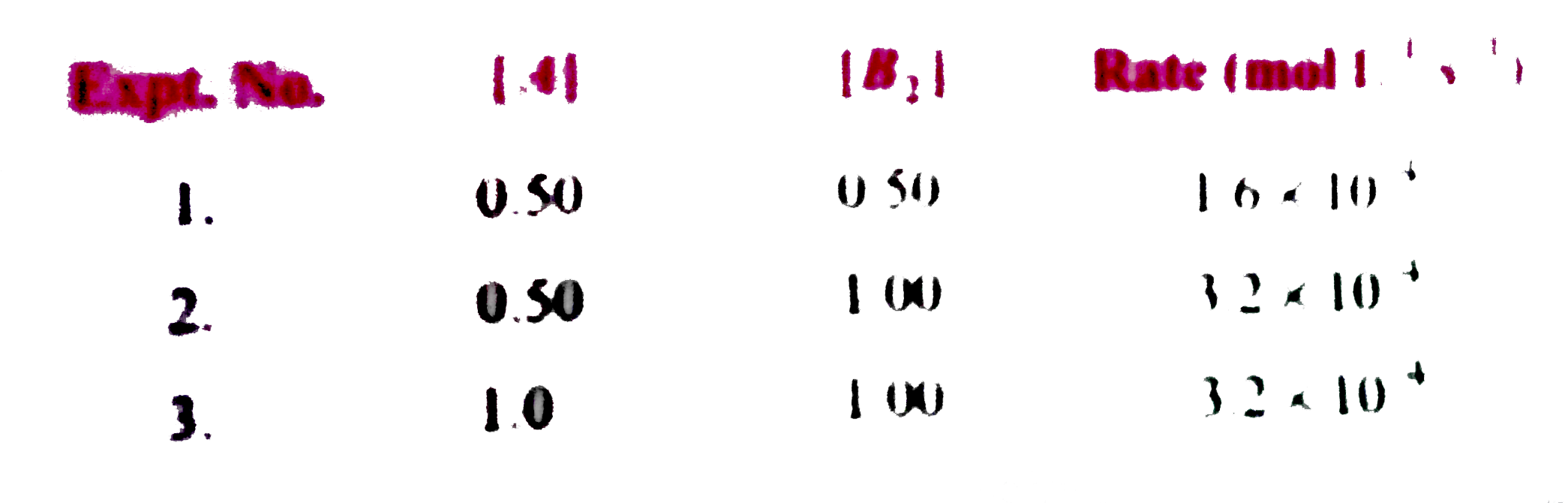
The rate equation for the above data is :
rate = k[B]2
rate = k[B]
rate =k[A]2[B]2
rate =k[A]2[B]
The correct Answer is:B
Step by step video, text & image solution for The experimental data for the reaction 2A+B_(2)rarr2AB, is : The rate equation for the above data is : by Chemistry experts to help you in doubts & scoring excellent marks in Class 12 exams.
Updated on:21/07/2023Similar Questions
Knowledge Check
The experimental data for the reaction 2A+B2→2AB is
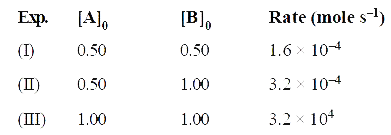
The rate equation for the above data is:ARate =K[B2][A]^2BRate =K[B2]2CRate =K[A]2[B]2DRate =K[A]2[B]The experiment data for the reaction 2A+B2→2AB is
∣∣ ∣ ∣ ∣ ∣∣Experiment[A]M[B2]MInitial rate(molL−1s−1)I0.500.51.6×10−4II0.501.03.2×10−4III1.001.03.2×10−4∣∣ ∣ ∣ ∣ ∣∣
Write the most probable rate equation for the reacting giving reason for you answer.ARate = k[A]2[B]2BRate = k[A]2[B]CRate = k[B2]DRate = [B2]2The experiment data for the reaction 2A+B2→2AB is
∣∣ ∣ ∣ ∣ ∣∣Experiment[A]M[B2]MInitial rate(molL−1s−1)I0.500.51.6×10−4II0.501.03.2×10−4III1.001.03.2×10−4∣∣ ∣ ∣ ∣ ∣∣
Write the most probable rate equation for the reacting giving reason for you answer.Arate = K[A]2[B]2Brate = K[A]2[B]Crate = k[B2]Drate = k[B2]2
The data for the reaction A+B→C is
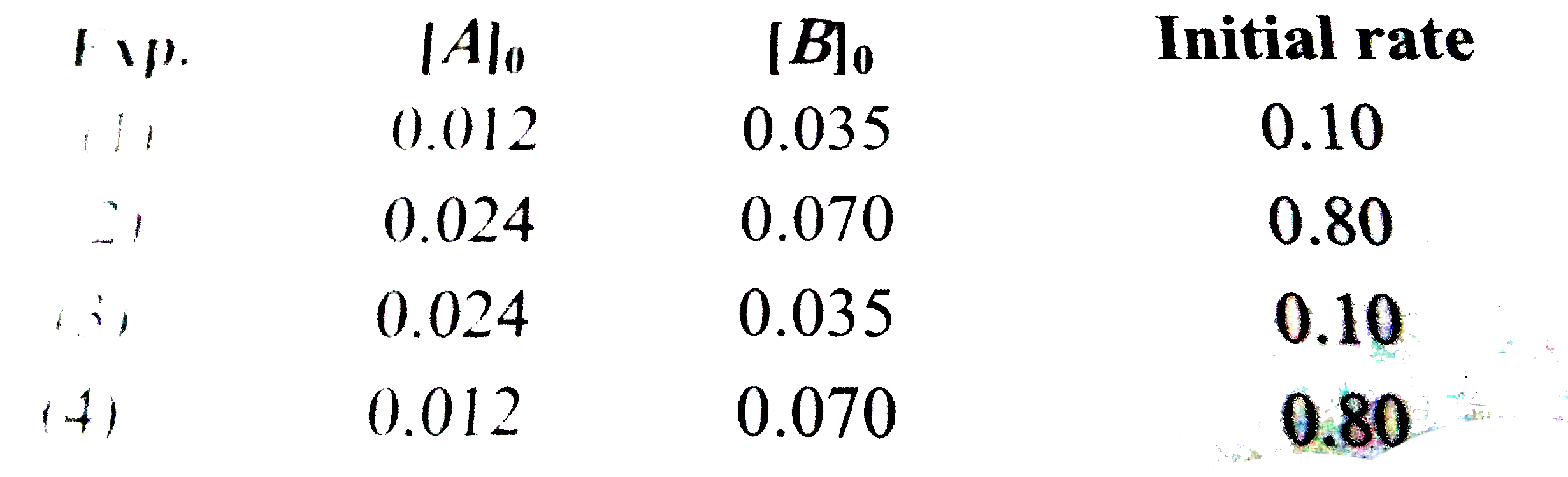
The rate law corresponds to the above data is
View SolutionThe experiment data for the reaction 2A+B2→2AB is
∣∣ ∣ ∣ ∣ ∣∣Experiment[A]M[B2]MInitial rate(molL−1s−1)I0.500.51.6×10−4II0.501.03.2×10−4III1.001.03.2×10−4∣∣ ∣ ∣ ∣ ∣∣
Write the most probable rate equation for the reacting giving reason for you answer.
View SolutionUse the experimental data in the table to determine was studies
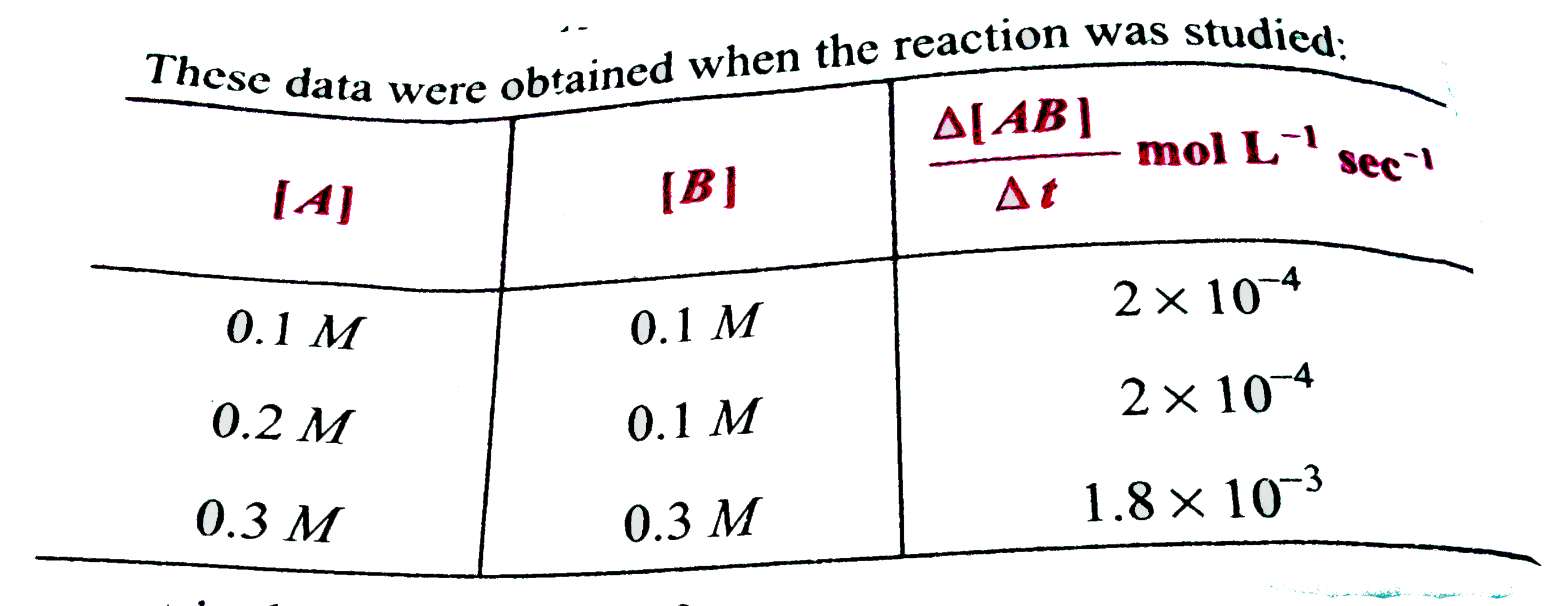
What is the rate equation for the reaction ?
View SolutionThe following graph is experimentally obtained for the reaction : A→2B, at 25∘C. The correct statement (s) for the reaction is/are (Given : ln 2 = 0.7)
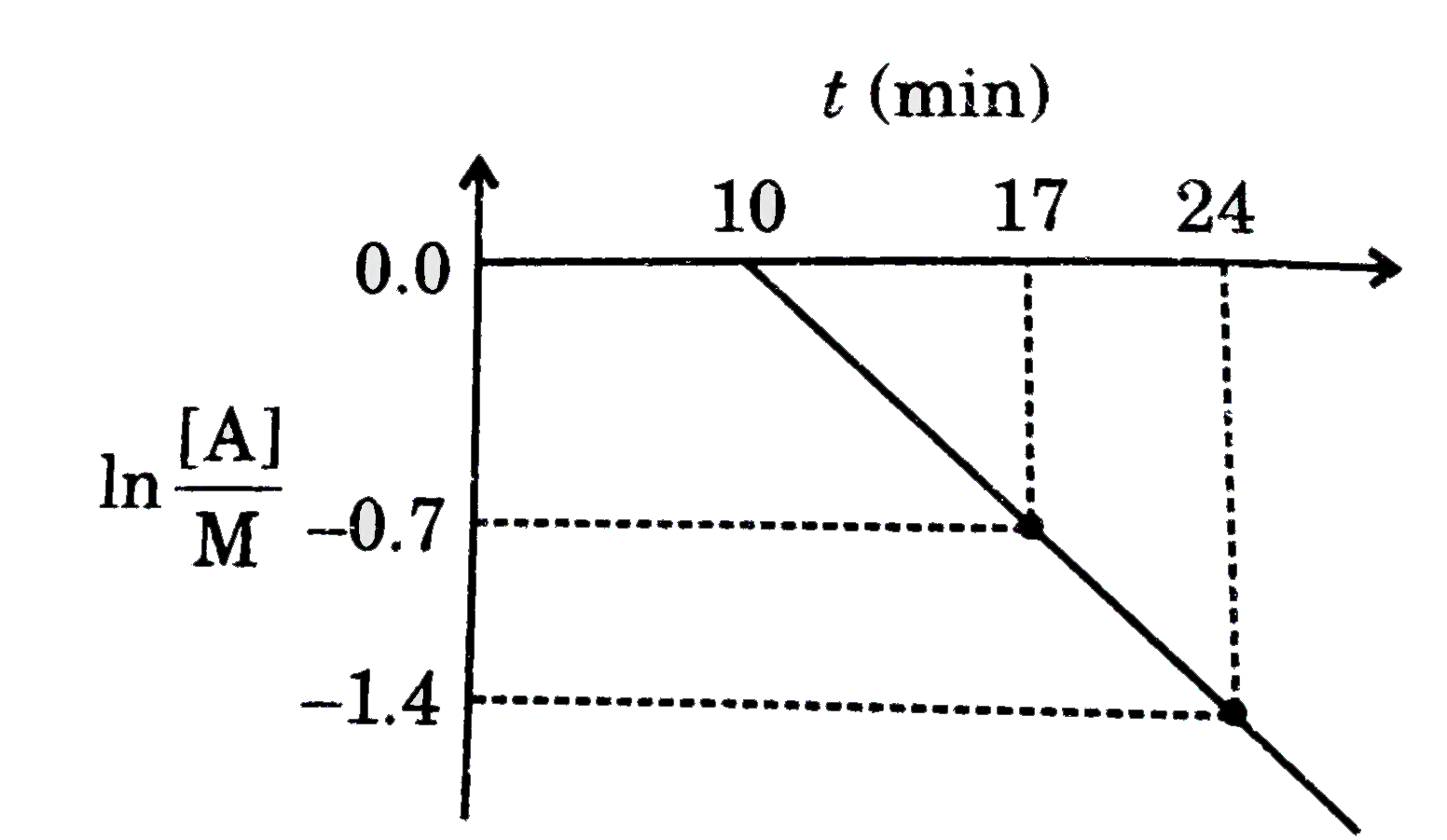
View SolutionFor a reaction A2+B2→2AB, evaluate the energy of activation from the following data :

View Solution