In which of the following reactions, the increase in the rate of reaction will be maximum?
A
EaTemperature rise(a)40kJ/mol200−210K
B
EaTemperature rise(b)90kJ/mol300−320K
C
EaTemperature rise(c)80kJ/mol300−310K
D
All will have same rate
Video Solution
Text Solution
The correct Answer is:B
|
Answer
Step by step video, text & image solution for In which of the following reactions, the increase in the rate of reaction will be maximum? by Chemistry experts to help you in doubts & scoring excellent marks in Class 12 exams.
Updated on:21/07/2023Similar Questions
Knowledge Check
A catalyst increases the rate of reaction by :
Adecreasing the free energy change for reaction.Bdecreasing the enthalpy change of reaction.Cdecreasing the activation energy for reaction.Dall of the aboveAn enzyme increases the rate of a reaction by
Asupplying the energy requried to start the reactionBincreasing the rate of random collisions of moleculesCremoving the product of the reaction so allowing it to continueDbringing the reacting molecules into precise orientation with each otherEnzymes enhance the rate of reaction by
ABy lowering activation energyBBy changing activation energyCBy changing equilibrium constantDBy forming enzyme substrate complex
Rank the following in the increasing oprder of rate of reaction with HBr
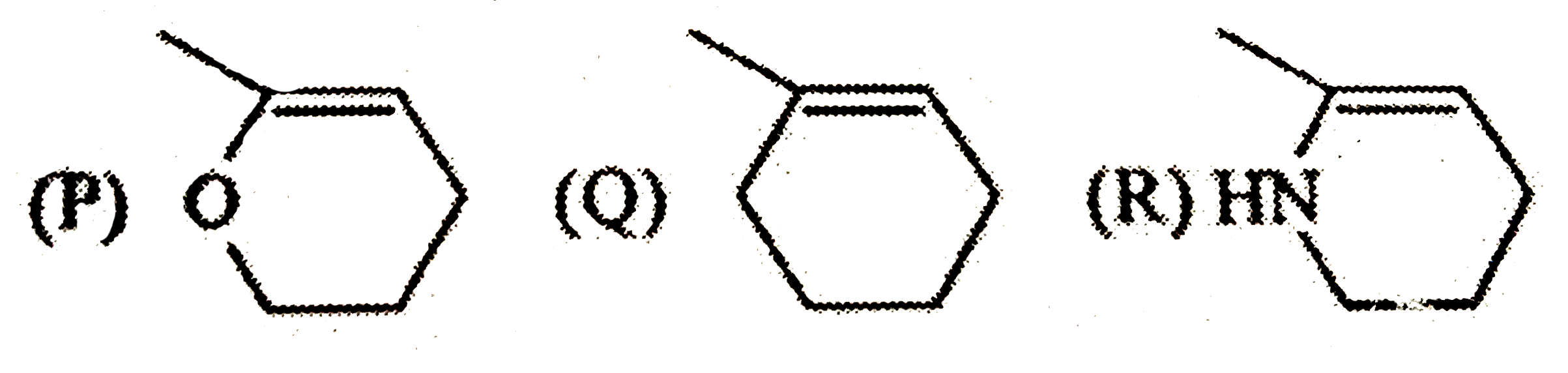
View SolutionFor which of the following reactions, the units of rate constant and rate of reaction are same?
View SolutionWhich of the following explains the increase of the reaction rate by catalyst?
View SolutionWith increase in temperature , rate of reaction
View SolutionA catalyst increases the rate of a reaction by:
View Solution