What happens when ? Give equations only:
(i) chlorine reacts with CS2 in pressure of anhydrous aluminium chloride .
(ii) Chloride is heated with alcoholic KOH and aniline.
(iii) Ethyl alcohol is heated with ionic and sodium hydroxide.
(iv) Ethyl alcohol reacts with bleaching powder.
(v) Chloride is passed through ethyl alcohol.
(vi) Ethyl bromide reacts with sodium ethoxide.
(vii) Ethyl bromide reacts with silver cyanide.
(viii) Chloral is treated with aqueous sodium hydroxide.
(ix) Carbon tetrachloride is treated with hydrogen fluoride in pressure of antimony fluoride.
(x) Ethyl amine is heated with chloroform and alcoholic KOH.
(xi) Chloroform is boiled with aqueous potassium hydroxide.
(xii) Ethyl bromide is treated with alcoholic caustic soda.
(xiii) Ethyl bromine is heated with zinc.
(xiv) Methyl magnesium bromide is treated with formaldehyde followed by hydrolysis.
(xv) 2-Bromopentane is heated with alcoholic KOH.
(xvi) 2-Chloro-2- methylpropane is treated with potassium tert-butoxide in dimethyl sulphoxide.
(xvii) tertiary Butyl chloride reacts with ethylene in presence of AICI3
(xviii) Benzene reacts with n-propyl chloride in pressure of anhydrous AICI3
Text Solution
(i)CS2+3CI2AICI−−→CCI4+S2CI2(i)CS2+3CI2AICI−−→CCI4+S2CI2
(ii)C6H5NH2+CHCI3+3KOH→C6H5NC+3KCI+3H2O
(iii)C2H5OH+4I2+6KOH→CHI3+HCOOK+5KI+5H2O
(iv)CaOCI2+H2O→Ca(OH)2+CI2,C2H5OHCI2−−−−−→[O]
CH3CHOCI2−−→CCI3CHOCa(OH)2−−−−−→CHCI3+(HCOO)2Ca
(v)C2H5OHCI2−−−−−→[O]CH3CHOCI2−−→CCI3CHO(Chloral)
(vi)C2H5Br+NaOC2H5→C2H5OC2H5+NaBr
(vii)C2H5Br+AgCN→C2H5NC+AgBr
(viii)CCI3CHO+NaOH→CHCI3+HCOONa
(ix)CCI4+H2F2SbF2−−→CCI2(Freon)F2+2HCI
(x)C2H5NH2+CHCI3+3KOH→C2H5NC+3KCI+3H2O
(xi)CHCI3+4KOH→HCOOK+3KCI+2H2O
(xii)C2H5Br+alc.KOH→C2H4+KBr+H2O
(xiii)2C2H5Br+2Zn→(C2H5)2Zn+ZnBr2
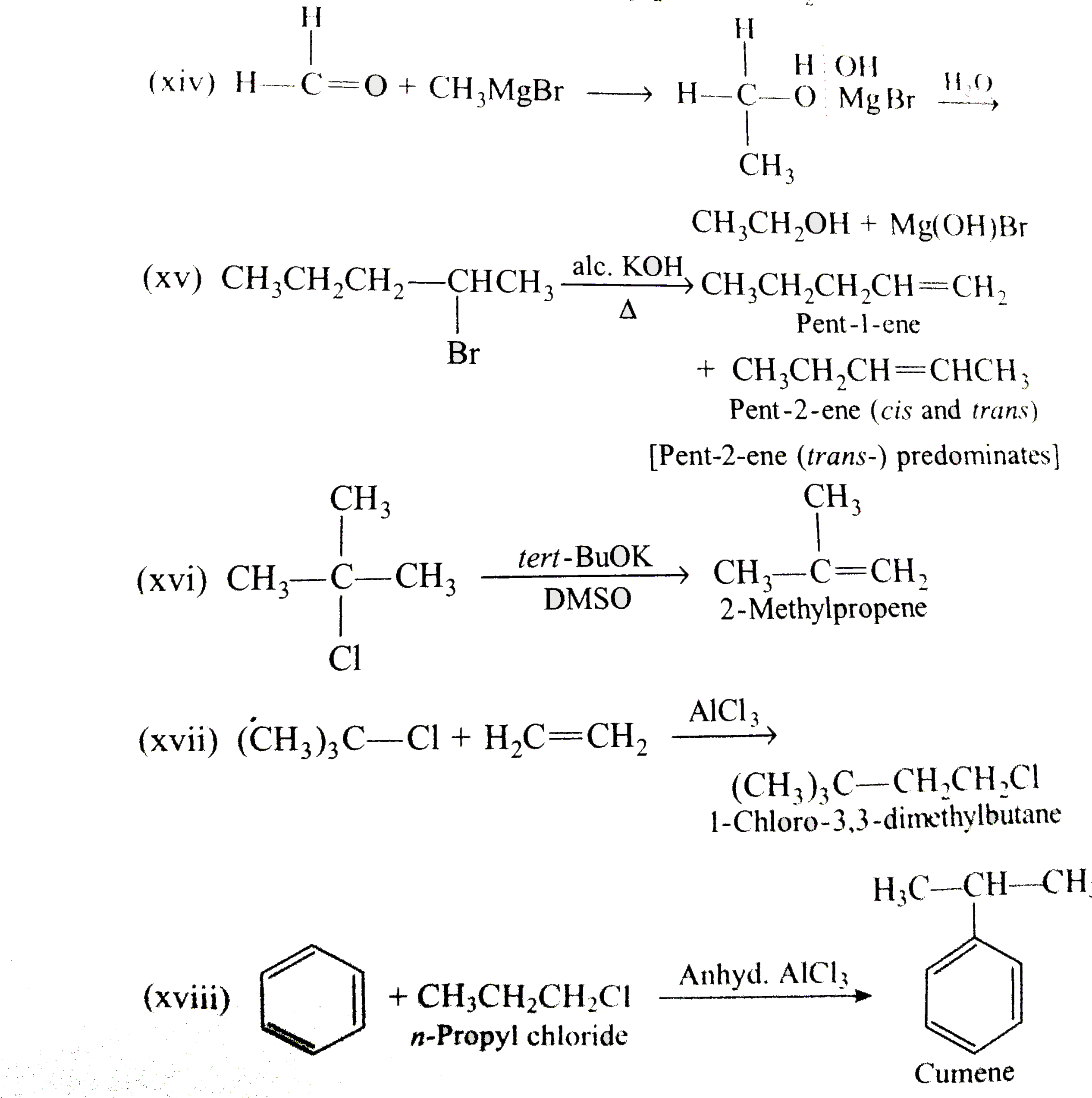
|
Similar Questions
Knowledge Check
Ethyl chloride react with sodium ethaoxide given,
Aethoxy ethaneBmethoxy ethaneCethaneDn-butaneEthyl alcohol reacts with acetyl chloride to form
AEthyl chlorideBAcetic acidCMethyl acetateDEthyl acetateEthyl alcohol is reacted with acetyl chloride gives
Aethyl acetateBethyl formateCethyl propanoateDethyl methyl ketone
Ethyl alcohol reacts with sodium with evolution of H2 gas. Ethyl alcohol is
View SolutionWhat happens when:
(i). Methyl chloride is treated with alcoholic KCN.
(ii). Ethyl chloride is treated with alcoholic KOH.
(iii). Chloroform is heated with Ag powder.
View SolutionEthyl bromide can be converted into ethyl alcohol by
View SolutionWhen ethyl alcohol is reacted with PCl5 gives
View SolutionEthyl alcohol gives ethyl chloride with the help of
View Solution

