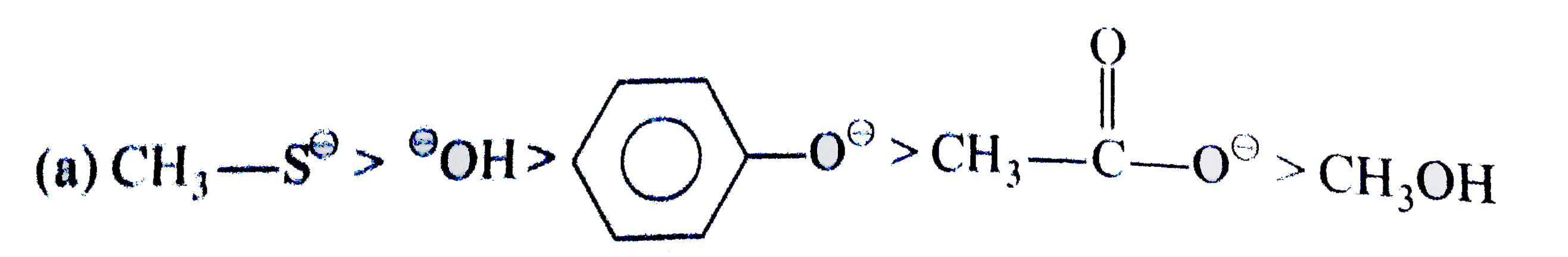
(a) List the following species in order or decreasing nucleophilicity in aqueous medium.

(b) Indicate which of the following solvent is protic or aprotic . ,
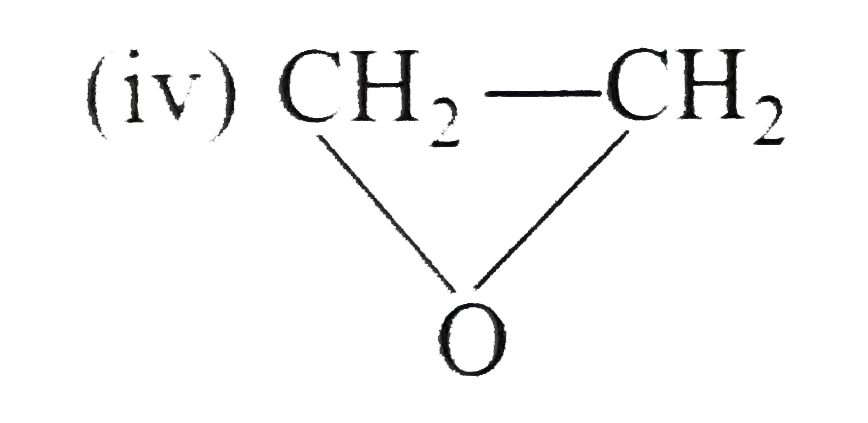
(C) Identify the strongest nucleophile in each pair of anions.
(i) BrΘ and CIΘ in H2O(ii).ΘSH and FΘ in CH3OH
(iii),ΘOH and CIΘ in DMF
Text Solution
(b)protic−(i),(iii),(v)
Aprotic−(ii),(iv),(vi)
(C)(i)BrΘ(ii).ΘSH(iii).ΘOH
|
Similar Questions
Knowledge Check
Rank the following species in order of decreasing nucleophilicity in a polar protic solvent (most → least nucleophilic):
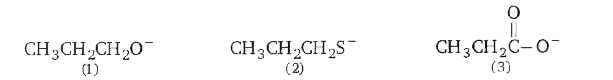 A3 gt 1 gt 2B2 gt 3 gt 1C1 gt 3 gt 2D2 gt1 gt 3
A3 gt 1 gt 2B2 gt 3 gt 1C1 gt 3 gt 2D2 gt1 gt 3Strongest nucleophile in polar-protic solvent is
ACH3O−BCH3SHCCH3−S−DCH3OHIn the reaction I2+2OHΘ→IΘ+IOΘ+H2OI2 is
AOxidisedBReducedCOxidised and reducedDForms complex
Complete and balance the following reactions: CIOΘ3+IΘ+H2SOΘ4→CIΘ+HSOΘ4+…….
View SolutionComplete and balance the following reactions: CIOΘ3+IΘ+H2SOΘ4→CIΘ+HSOΘ4+…….
View SolutionIn the reaction I2+2OHΘ→IΘ+IOΘ+H2OI2 is
View SolutionWhat is the correct order of decreasing nucleophilicity in non-polar solvents: OH−,NH−2,F−,CH−3
View SolutionWhat is the correct order of decreasing nucleophilicity in non-polar solvents: OH−,NH−2,F−,CH−3
View Solution

