Alexander Williamson prepared diethyl by a simple method, now called as Williamson's ether synthesis . In this method, an alkyl halide is treated with sodium alkoxide prepared from sodium and alcohol.
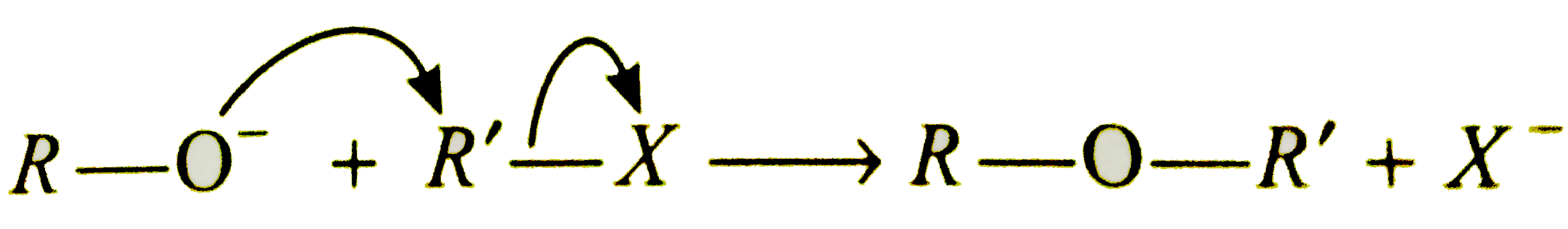
This reaction is used in the synthesis of symmetrical and unsymmetrical ethers.
It may be noted taht for preparing unsymmetrical ethers, the halide used should preferably be primary because secondary and tertiary alkyl halides may form alkenes as major product due to elimination process.
CH3−CH3∣C∣CH3−Br+Na+O−−C2H5Sodium ethoxide→CH3−CH3∣C=CH22-methylpropene+C2H5OH+NaBr
Aryl ethers or phenolic ethers can be prepared by using sodium phenoxide and alkyl halides. However, aryl halides and sodium alkoxide cannot be used for preparing phenolic ethers because aryl halides are less reactive towards nucleophilic substitution reaction
Methyl tertiary butyl ether (MTBE) is added in gasoline to improve its octane number .
CH3−CH3∣C∣CH3−O−CH3
Which of the following is the best method for synthesis of the above ether ?
(Ch3)2C=CH2+CH3OHH2SO4−−−→
(CH3)3CBr+CH3ONa→
(CH3)3C−O−Na+CH3I→
All of these reactions
The correct Answer is:C
Step by step video, text & image solution for Alexander Williamson prepared diethyl by a simple method, now called as Williamson's ether synthesis . In this method, an alkyl halide is treated with sodium alkoxide prepared from sodium and alcohol. This reaction is used in the synthesis of symmetrical and unsymmetrical ethers. It may be noted taht for preparing unsymmetrical ethers, the halide used should preferably be primary because secondary and tertiary alkyl halides may form alkenes as major product due to elimination process. CH_(3)-underset(CH_(3))underset(|)overset(CH_(3))overset(|)(C)-Br+underset("Sodium ethoxide")(Na^(+)O^(-)-C_(2)H_(5))rarrunderset("2-methylpropene")(CH_(3)-overset(CH_(3))overset(|)(C)=CH_(2))+C_(2)H_(5)OH+NaBr Aryl ethers or phenolic ethers can be prepared by using sodium phenoxide and alkyl halides. However, aryl halides and sodium alkoxide cannot be used for preparing phenolic ethers because aryl halides are less reactive towards nucleophilic substitution reaction Methyl tertiary butyl ether (MTBE) is added in gasoline to improve its octane number . CH_(3)-underset(CH_(3))underset(|)overset(CH_(3))overset(|)(C)-O-CH_(3) Which of the following is the best method for synthesis of the above ether ? by Chemistry experts to help you in doubts & scoring excellent marks in Class 12 exams.
Updated on:21/07/2023Similar Questions
In Williamsons synthesis t-alkyl halide can not be used for preparation of alkyl t-butyl ether, because
View SolutionEthers can be prepared by Williamson synthesis in which an alkyl halide is reacted with sodium alkoxide. Di-tertiary ether can't be prepared by this method. Explain.
View SolutionEthers can be prepared by Williamson synthesis which an alkyl halide is reacted with sodium alkoxide. Di-tert-butyl ether can't be prepared by this method. Explain.
View SolutionTertiary alkyl halides are not suitable for preparation of ethers by Williamson's synthesis, Explain.
View SolutionEthers can be prepared byWilliamson synthesis in which an alkyl halide is reacted with sodium alkoxide. Di - tert butyl ether cannot be prepare by this method. Explain.
View Solutionईथरों को विलियम्सन संश्लेषण द्वारा प्राप्त किया जा सकता है जिसमें ऐल्किल हैलाइडों को सोडियम ऐल्कॉक्साइड से अभिकृत किया जाता है। इस विधि से डाइ-टर्शरी-ब्युटिल ईथर नहीं बनाई जा सकती। स्पष्ट कीजिए क्यों?
View SolutionThe reaction, Sodium alkoxide+Alkyl halide rarr Ether+Sodium halide is called
View SolutionEthers can be prepared by Williamson synthesis which an alkyl halide is reacted with sodium alkoxide. Di-tert-butyl ether can't be prepared by this method. Explain.
View Solution
