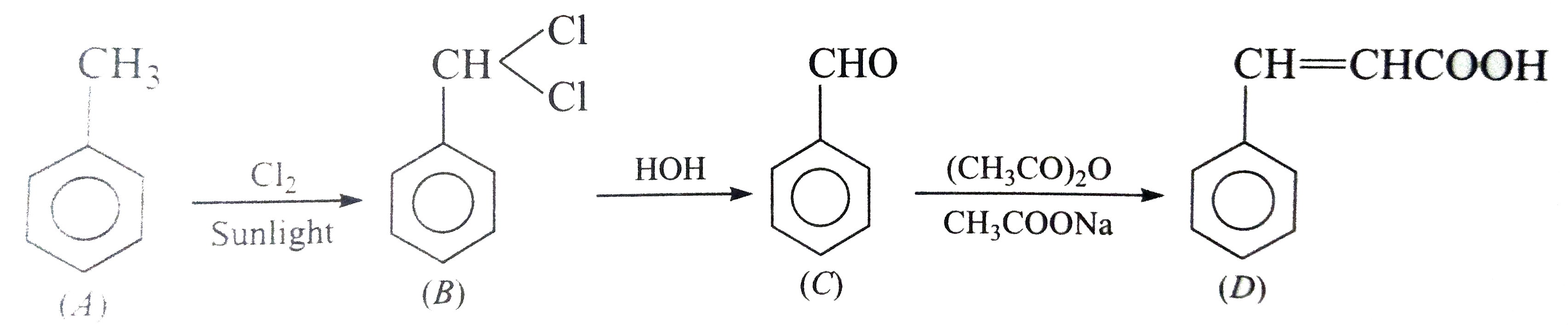
Compound (A), having an empirical fomrula C7H8, is chlorinated in sunlight to give a product (B), Which on hydrolysis gives a compound (C).(C) gives a positive test with Tollens reagen and with sodium, acetate and acetic anhydride gives an acid (D) , with equivalent weight 148.15 . Identify (A),(B) (C) and (D) adn explain the reactions involved.
Topper's Solved these Questions
- ALDEHYDES AND KETONES BOOK - OP TANDONCHAPTER - ALDEHYDES AND KETONES EXERCISE - BRAIN STORMIN PROBLEMS
13 Videos
- ALDEHYDES AND KETONES BOOK - OP TANDONCHAPTER - ALDEHYDES AND KETONES EXERCISE - OBJECTIVE QUESTIONS (LEVEL -A)
254 Videos
- ALDEHYDES AND KETONES BOOK - OP TANDONCHAPTER - ALDEHYDES AND KETONES EXERCISE - MATCHING TYPE
1 Video
- ALCOHOLS, PHENOLS AND ETHERSBOOK - OP TANDONCHAPTER - ALCOHOLS, PHENOLS AND ETHERSEXERCISE - SINGLE INETER ANSWER TYPE QUESTIONS / ALCOHOLS AND PHENOLS
15 Videos
- BASIC PRINCIPLESBOOK - OP TANDONCHAPTER - BASIC PRINCIPLESEXERCISE - SECTION V INTEGER ANSWER TYPE QUESTION
3 Videos
Similar Questions
A compound (A) of boron reacts with Nme3 to give an adduct (B) which on hydrolysis gives a compound (C) and hydrogen gas. Compound (C) is an acid. Identify the compounds A,B and C. give the reactions inovolved.
View SolutionA compound (A) of boron react with NMe3 to give an adduct (B) which in hydrolysis gives a compound (C) and hydrogen gas. Compound (C) is an acid. Indenfity the compounds A,B and C. Give the reactions involved.
View Solution
Knowledge Check
Compound C4H8CI2(A) on hydrolysis gives a compound C4H8O(B) which reacts with hydroxylamine and does not give any test with Tollens reagent . What are (A) and (B) ?
A1,1-Dichlorobutane and butanalB2,2-Dichlorobutane and butanalC1,1-Dichlorobutane and butan-2-oneD2,2-Dichlorobutane and butan-2-oneAn compound (A ) C4H8Cl2 on alkaline hydrolysis to give compound (B ) C4H8O which gives an oxime and positive Tollen's reagent test . What is the structure of (A) ?
ACH3CH2CH2CHCl2BCH3CCl2CH2CH3CCH3CH(Cl)CH(Cl)CH3DCH2ClCH2CH2CH2Cl
A compound (A) of boron reacts with Nme3 to give an adduct (B) which on hydrolysis gives a compound (C) and hydrogen gas. Compound (C) is an acid. Identify the compounds A,B and C. give the reactions inovolved.
View SolutionAn organic compound A with molecular formula C2H7N on reaction with nitrous acid gives a compound B.B on controlled oxidation gives a compound C. C reduces Tollens' reagent to give silver mirror and D. B reacts with D in the presence of concentrated sulfuric acid to give a sweet smelling compound E. Identify A, B, C, D and E. Give the reaction of C with ammonia and name the product.
View SolutionCompound (A) and (B) are isomers having C8H10. On oxidation (A) gives benzoic acid while (B) gives phthalic acid which forms an anhydride (C) on heating. Identify (A), (B) and (C).
View Solution(i) An organic compound A with molecular formula C7H8 on oxidation by chromyl chloride in the presence of CCl4 gives a compound B which gives positive Tollen's test. The compound B on treatment with NaOH followed by acid hydrolysis gives two product C and D. C on oxidation gives B which on further oxidation gives D. the compound D on distillation with sodalime gives a hydrocarbon E. Below 60∘C, concentrated nitric acid reacts with E in the presence of concentrated sulphuric acid forming a compound F. identify the compounds A,B,C,D,E and F.
(ii) Give chemical test to distinguish : Formaldehyde and acetaldehyde.
View SolutionKetone (A), which indergoes halform reaction, give compound (B) on reduction. (B) on heating with H2SO4 gives compound (C), which forms mono-ozonide (D).(D) on hydrolysis in the presence of Zn dust gives only acetaldehyde. Identify (A).(B), and (C). Write down the reactions involved.
View SolutionAcetoisonitrile on reaction with C2H5MgBr followed by hydrolysis, gives compound (A), which one further hydrolysis gives (B) and (C).
(B) and (C) are :
View Solution