Among the following compounds, which one has the shortest C−Cl bond?
H3C−Cl
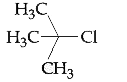

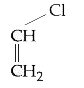
The correct Answer is:D
To determine which compound has the shortest C-Cl bond among the given options, we need to analyze the nature of the bonds in each compound. Here's a step-by-step solution:
Step 1: Identify the Compounds
First, we need to identify the compounds provided in the question. Let's assume the compounds are:
1. CH3Cl (Methyl chloride)
2. CH2=CHCl (Vinyl chloride)
3. C6H5Cl (Chlorobenzene)
4. CHCl3 (Chloroform)
Step 2: Analyze the Bond Types
Next, we analyze the type of bonds present in each compound:
- CH3Cl: Contains a single C-Cl bond.
- CH2=CHCl: Contains a C-Cl bond, but the carbon is involved in a double bond with another carbon (C=C).
- C6H5Cl: The C-Cl bond is attached to a carbon in a benzene ring, which has resonance.
- CHCl3: Contains a single C-Cl bond.
Step 3: Consider Bond Length Trends
In general, the bond length decreases with increasing bond order:
- Single bonds (C-Cl) are longer than double bonds (C=C) and triple bonds (C≡C).
- However, resonance and hybridization can affect bond lengths as well.
Step 4: Evaluate the Compounds
1. CH3Cl: The C-Cl bond length is relatively long due to the single bond.
2. CH2=CHCl: The C-Cl bond may have some double bond character due to the adjacent double bond, which can shorten the bond length.
3. C6H5Cl: The C-Cl bond is affected by resonance in the benzene ring, which can also lead to a shorter bond length compared to a simple single bond.
4. CHCl3: Similar to CH3Cl, the C-Cl bond is a single bond and relatively long.
Step 5: Conclusion
Among these compounds, CH2=CHCl (Vinyl chloride) is expected to have the shortest C-Cl bond due to the presence of the adjacent double bond, which imparts some double bond character to the C-Cl bond.
Final Answer
The compound with the shortest C-Cl bond is CH2=CHCl (Vinyl chloride).
---
First, we need to identify the compounds provided in the question. Let's assume the compounds are:
1. CH3Cl (Methyl chloride)
2. CH2=CHCl (Vinyl chloride)
3. C6H5Cl (Chlorobenzene)
4. CHCl3 (Chloroform)
Next, we analyze the type of bonds present in each compound:
- CH3Cl: Contains a single C-Cl bond.
- CH2=CHCl: Contains a C-Cl bond, but the carbon is involved in a double bond with another carbon (C=C).
- C6H5Cl: The C-Cl bond is attached to a carbon in a benzene ring, which has resonance.
- CHCl3: Contains a single C-Cl bond.
In general, the bond length decreases with increasing bond order:
- Single bonds (C-Cl) are longer than double bonds (C=C) and triple bonds (C≡C).
- However, resonance and hybridization can affect bond lengths as well.
1. CH3Cl: The C-Cl bond length is relatively long due to the single bond.
2. CH2=CHCl: The C-Cl bond may have some double bond character due to the adjacent double bond, which can shorten the bond length.
3. C6H5Cl: The C-Cl bond is affected by resonance in the benzene ring, which can also lead to a shorter bond length compared to a simple single bond.
4. CHCl3: Similar to CH3Cl, the C-Cl bond is a single bond and relatively long.
Among these compounds, CH2=CHCl (Vinyl chloride) is expected to have the shortest C-Cl bond due to the presence of the adjacent double bond, which imparts some double bond character to the C-Cl bond.
The compound with the shortest C-Cl bond is CH2=CHCl (Vinyl chloride).
Similar Questions
Knowledge Check
Among the following compounds, which one is not an ether?
A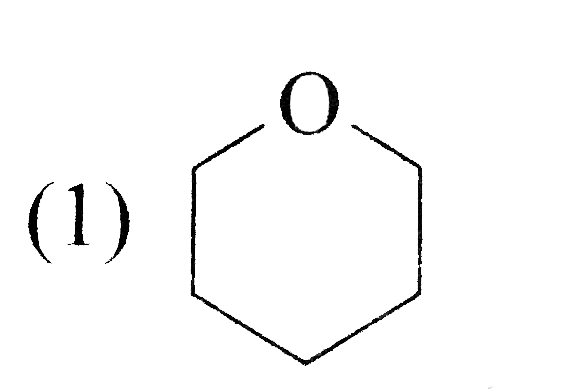 B
B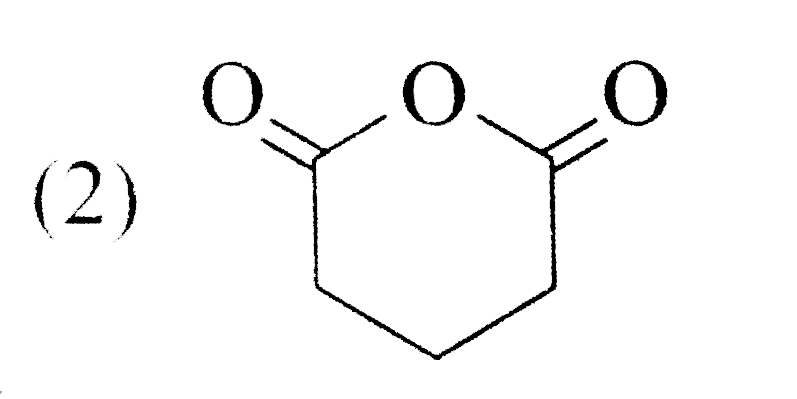 C
C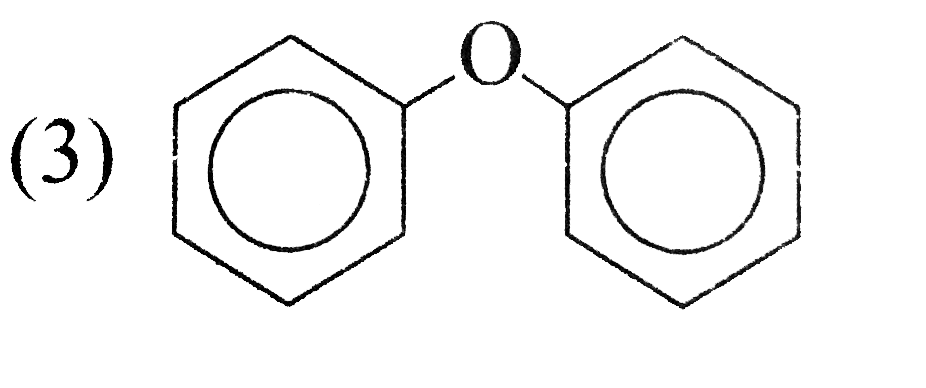 D
D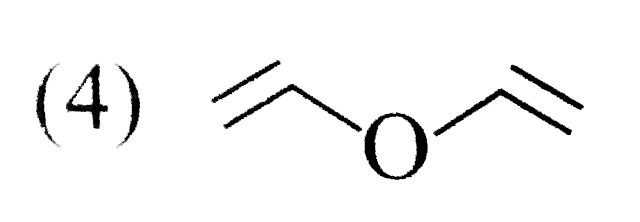
Among the following compounds, which has a dipole moment
ACCl4BC6H6CBF3DHFAmong the following organisms which one has the shortest life span?
AElephantBGiant TortoiseCSwanDParrot
Among the following isostructural compounds which one has highest lattice energy ?
View SolutionWhich of the following molecules contains the shortest C−H bonds?
View SolutionWhich one among the following compounds has the highest dipole moment ? .
View SolutionAmong the following compounds which one will react with acetone to give a product containing a carbon-nitrogen double bond ?
View SolutionWhich of the following compounds has the strongest hydrogen bonding?
View Solution

