The product of the reaction between one mole of XeO3 and two mole of XeF6 is :
A
XeO2F2
B
XeOF4
C
XeO3F2
D
XeO4
Text Solution
The correct Answer is:B
|
Similar Questions
Knowledge Check
The product of reaction between one mole of acetylene and two mole of HCHO in the presence of Cu2Cl2
AHOCH2−C≡C−CH2OHBH2C=CH−C≡C−CH2OHCHC=C−CH2OHDNone of theseThe product of the reaction between phenyl benxoate and one mole of bromine in the presence of iron is
A B
B C
C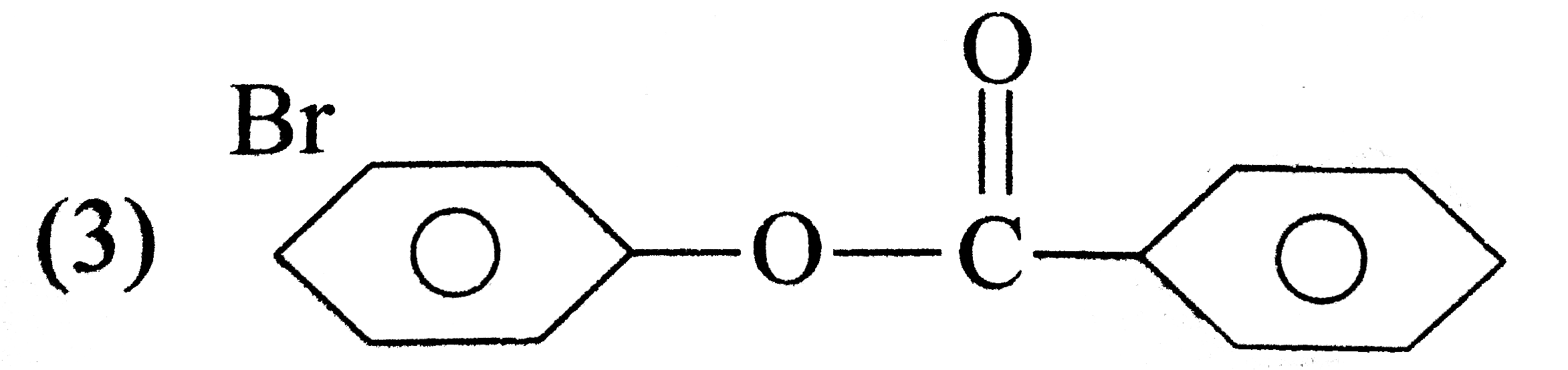 D
D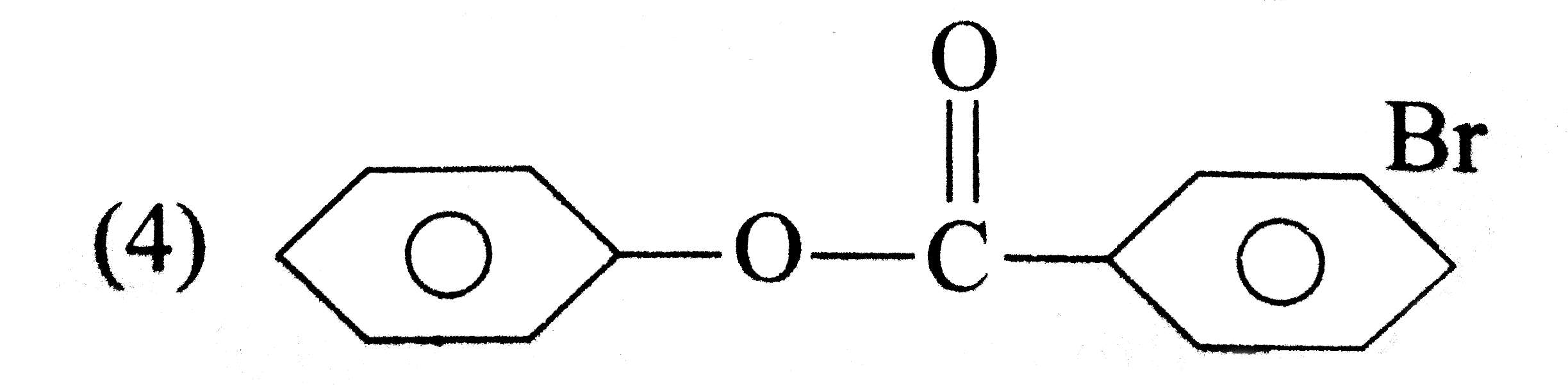
One mole of Co(NH3)5Cl3 gives 3 moles of ions on dissolution in water. One mole of this reacts with two moles of AgNO3 to give two moles of AgCl. The complex is :
A[Co(NH3)4Cl2]Cl.NH3B[Co(NH3)4Cl]Cl2.NH3C[Co(NH3)5Cl]Cl2D[Co(NH3)3Cl3].2NH3
Q31.The products formed in the following reaction at 873K and 6−7 bar [Xe+F2→? are : ,[(1:5)], (a) XeF4, (b) XeF6, (c) XeF6, (d) XeO2F4]
View SolutionIn case of XeO2F2 and XeF6 , Xe is with
View SolutionIn XeO3 and XeF6 the oxidation state of Xe is
View SolutionIn the reaction 4A+2B+3C→A4B2C3, what will be the number moles of product formed, starting from one mole of A, 0.6 moles of B and 0.72 moles of C ?
View SolutionOne mole of the complex CoCl3.6H2O on reaction with excess of AgNO3 agives two moles of white precipitate thus , complex is
View Solution

