Why is the enthalpy of sublimation equal to the sum of enthalpy of fusion and enthalpy of vaporisation ?
Video Solution
Text Solution
The correct Answer is:Since, sublimation involves (##RESPHYCHMXIC03E01183_A01##) According to Hess's law, ΔH(f)+ΔH(v)=ΔH(s)
Since, sublimation involves 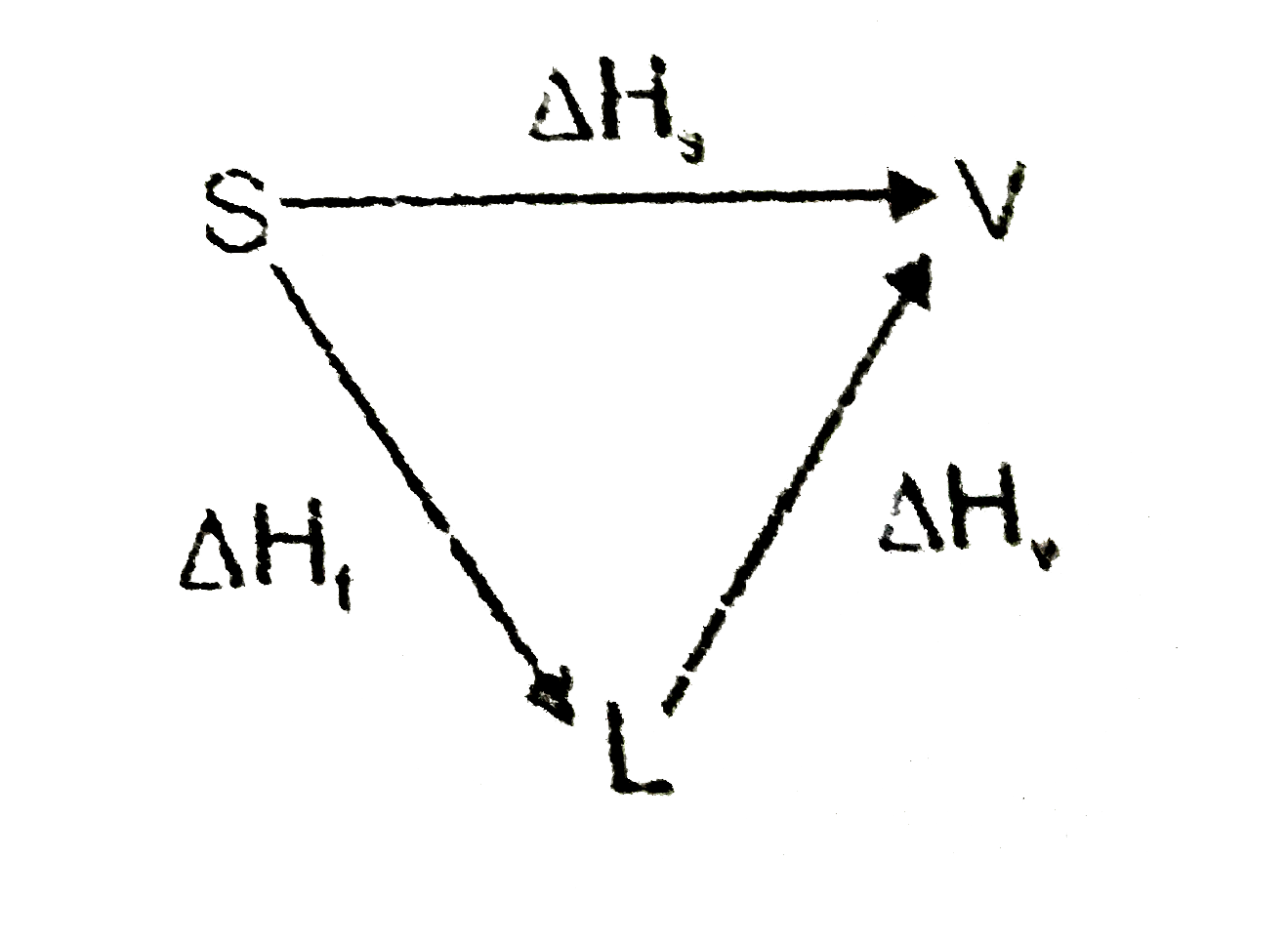
According to Hess's law, ΔH(f)+ΔH(v)=ΔH(s)
|
Similar Questions
Knowledge Check
Enthalpy of reaction is equal to
A∑ Bond of energies of reactants -∑ Bond of energies of products.B∑ Bond of energies of reactants + ∑ Bond of energies of products.C∑ Bond of energies of reactants ∑ Bond of energies of productDNone of theseEnthalpy of element is equal to the
AΔcH∘BΔrH∘CΔfH∘DAllEnthalpy of a compound is equal to its
Aheat of combustionBheat of formationCheat of solutionDheat of dilution
Define enthalpy of fusion.
View SolutionStandard enthalpy of reaction is equal to
View SolutionEnthalpy of a compound is equal to its :- (When it is formed from constituent particles)
View SolutionEnthalpy of sublimation of a substance is equal to :
View SolutionEnthalpy of sublimation of a substance is equal to
View Solution
