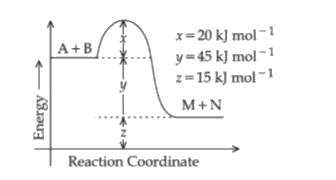
Look at the following diagram:
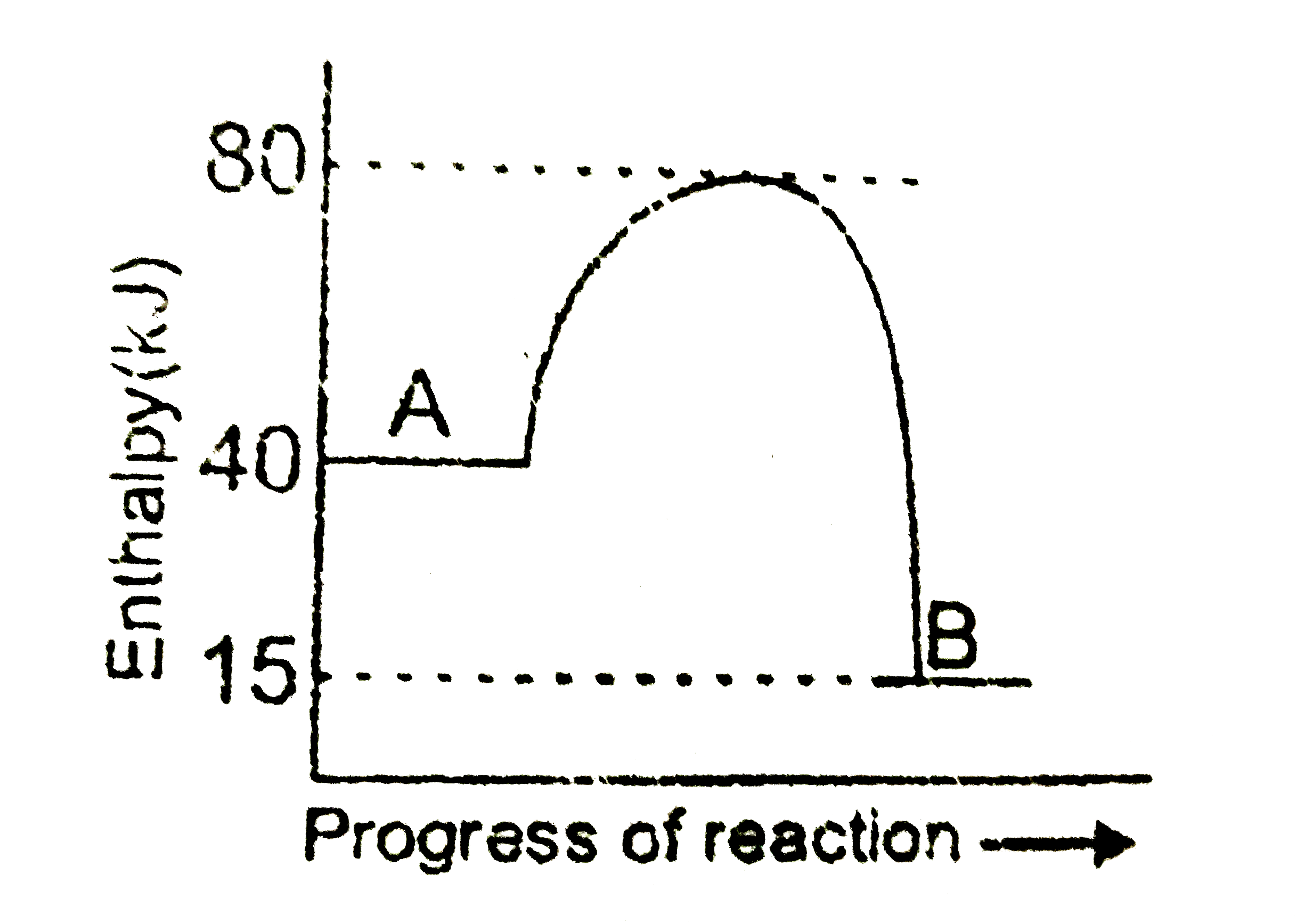
The enthalpy change for the reaction A→B will be
A
−25kJ
B
−40kJ
C
+25kJ
D
−65kJ
Video Solution
Text Solution
The correct Answer is:A
|
Answer
Step by step video, text & image solution for Look at the following diagram: The enthalpy change for the reaction A rarr B will be by Chemistry experts to help you in doubts & scoring excellent marks in Class 12 exams.
Updated on:21/07/2023Similar Questions
Knowledge Check
The enthalpy change of a reaction represents.
Achange in the concentration of the reactantsBdifference in the heat contents of the product and the reactantsCdifference in the number of moles of the reactants and productsDall of theseThe entalphy change for the reaction , H2Os→H2O(l) is called enthalpy of
AvapourizationBfusionCcombustionDtransitionThe enthalpy change for the process
C(graphite)→C(g)is calledAHeat of vaporisationBHeat fo sublimationCHeat of allotropic changeDHeat of atomisation
The enthalpy change for a reaction does not depend upon the
View SolutionThe enthalpy change for a reaction does not depend upon:
View SolutionThe enthalpy change of a reaction does not depand on
View SolutionThe enthalpy change of a reaction does not depend on
View SolutionThe enthalpy change for a reaction does not depend upon:
View Solution