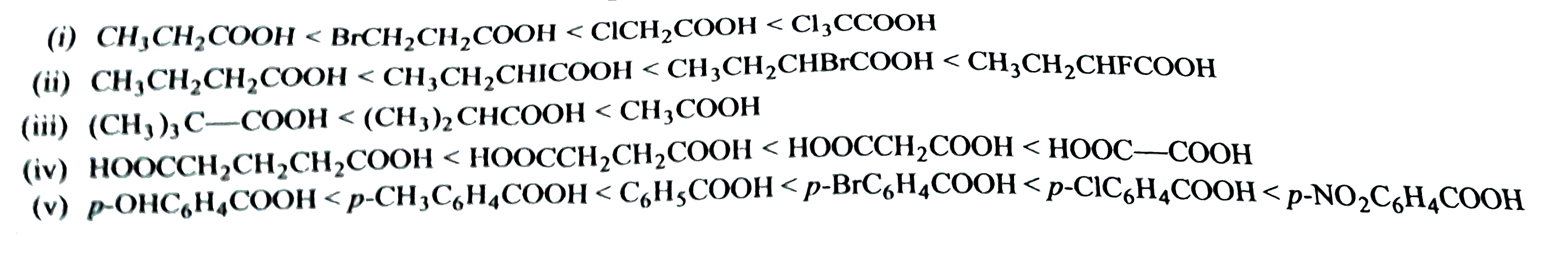Arrange the following in order of increasing acidity:
(i) Propanoic acid, chloroethanoic acid, 3-bromo-propanoic acid and trichloroacetic acid.
(ii) 2-Fluorobutanoic acid, 2-iodobutanoic acid, 2-bromobutanoic acid and butanoic acid.
(iii) (iii) Acetic acid, 2-methyl propanoic acid, 2,2-dimethyl propanoic acid.
(iv) Oxalic acid, malonic acid, succinic acid, glutaric acid.
(v) C6H5COOH,p−OHC6H4COOH,
p−CH3C6H4COOH,p−CIC6H4COOH,
p−BrC6H4COOH,p−NO2C6H4COOH.
Text Solution
|
Similar Questions
Knowledge Check
Rank the following compounds in increasing order of their acidity:
1. 3 - fluorobutanoic acid
2. 3 - chlorobutanoic acid
3. 2 - flurobutanoic acid
4. Butanoic acid
5. 4 - chlorobutanoic acid.A3<1<2<4<5B4<3<1<2<5C5<2<1<3<4D4<5<2<1<3Which of the following decreasing order of acid strength of
(i) Methanoic acid (ii) Ethanoic acid
(iii) Propanoic acid (iv) Butanoic acid
is correct ?A(i)>(ii)>(iii)>(iv)B(iv)>(iii)>(ii)>(i)C(i)>(iv)>(iii)>(ii)D(iv)>(i)>(ii)>(iii)Which of the following decreasing order of acid strength is correct?
I. Methanoic acid II. Ethanoic acid III. Propanoic acid IV. Butanoic acidAI gt II gt III gt IVBIV gt III gt I gt ICI gt IV gt III gt IIDIV gt I gt II gt III
Arrange the following in order of decreasing of acid strenth indicated ?
(i) Benzoic acid, 3, 4-Dinitrobenzoic acid, 4-Methylbenzoic acid ltbgt (ii) CH3CH2CH(Br)COOH,CH3CH(Br)CH2COOH,(CH3)2CHCOOH
View SolutionArrange the following in decreasing order of their acidic character:
(i) p−CH3O−C6H4OH,
(ii) p−NO2−C6H4OH,
(iii) C6H5OH
View SolutionConsider the acidity of the carboxylic acids :
(i)PHCOOH (ii)o−NO2C6H4COOH (iii)p−NO2C6H4COOH (iv)m−NO2C6H4COOH
View SolutionWhich is decreasing order of acidity in,
HCOOH(I),CH3COOH(II),CH3CH2COOH(III)andC6H5COOH(IV)?
View SolutionArrange given diabasic acids in decreasing order of acidic strength 1. Oxalic acid 2. Adipic acid 3. Malonic acid 4. Succinic acid
View Solution