Carboxylic acids readily dissolve in aqueous sodium bicarbonate, liberating carbon dioxide. Which one of the following is correct?
The conjugate base of the carboxyhc acid is more stable than the free carboxylic acid
Free carboxylic acid and its conjugate base are of comparable stability
The free carboxylic acid is more stable than its conjugate base
The conjugated acid of the carboxylic acid is more stable than the free carboxylic acid
The correct Answer is:A
Step by step video, text & image solution for Carboxylic acids readily dissolve in aqueous sodium bicarbonate, liberating carbon dioxide. Which one of the following is correct? by Chemistry experts to help you in doubts & scoring excellent marks in Class 12 exams.
Updated on:21/07/2023Similar Questions
Knowledge Check
Carboxylic acids readily dissolve in aqueous sodium bicarbonate, liberting carbon dioxide. Which one of the following is correct?
AFree carboxylic acid and its conjugate base are of comparable stabilityBThe free carboxylic acid is more stable than its conjugate baseCThe conjugate base of the carboxylic acid is more stable than the free carboxylic acidDAll of the aboveWhich of the following is soluble in aqueous sodium bicarbonate ?
A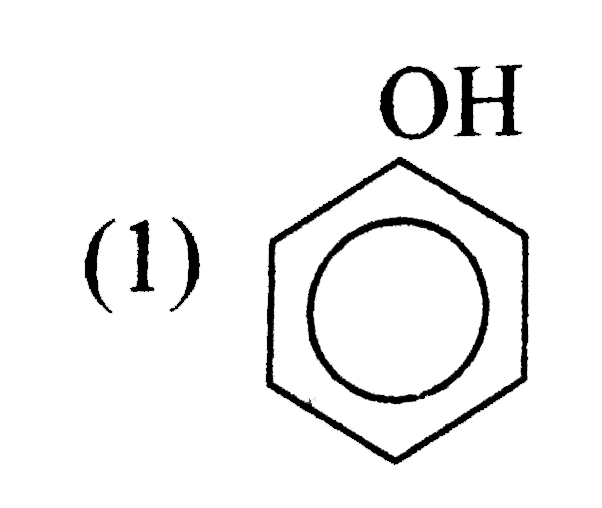 B
B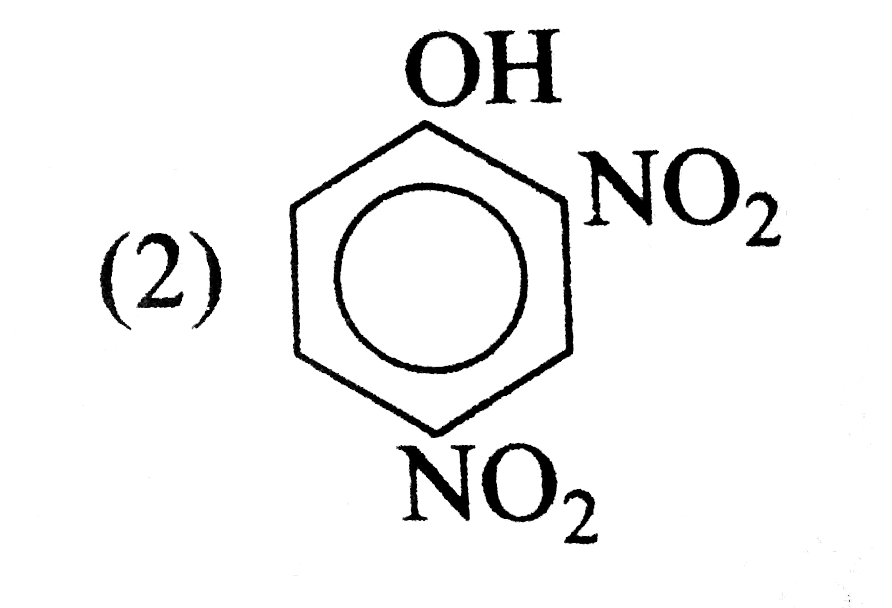 C
C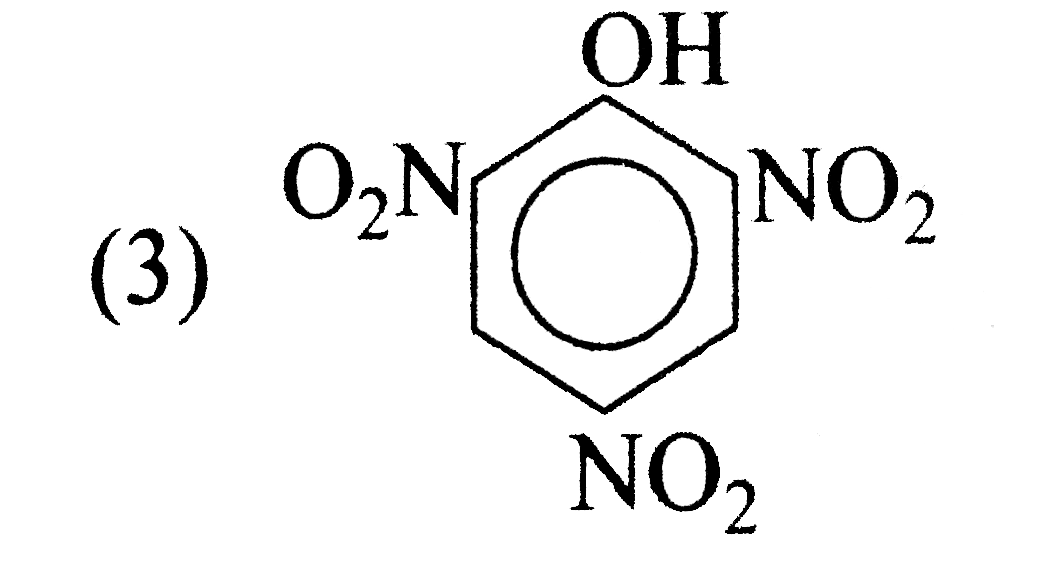 DBoth (2) and (3)
DBoth (2) and (3)Carboxylic acids dissolve in aqueous NaOH because acids undego
AProtonationBDeprotonationCCarboxylationDDecarboxylation
Sodium bicarbonate is chemical name of which of the following?
View SolutionWhen propionic acid is treated with aqueous sodium bicarbonate, CO2 is liberated. The 'C' of CO2 comes from
View SolutionWhen propionic acid is treated with aqueous sodium bicarbonate, CO2 is liberated. The C of CO2 comes form :
View SolutionWhen propionic acid is treated with aqueous sodium becarbonate, CO2 is liberated. The carbon of CO2 comes from
View SolutionWhen propionic acid is treated with aqueous sodium bicarbonate, CO2 is liberated. The 'C' of CO2 comes from
View Solution
