The intermediate(s) formed during the reaction
C6C5CH2COOAg+Br2CCl4−−→C6H5CH2Br, is (are):
C6H5−CH2−O∣∣−OBr
C6H5−CH2−O∣∣−∗O
C6H5−∗CH2
B∗r
The correct Answer is:A, B, C, D
Topper's Solved these Questions
- CARBOXYLIC ACIDS AND THEIR DERIVATIVESBook:OP TANDONChapter:CARBOXYLIC ACIDS AND THEIR DERIVATIVESExercise:Integer
Explore 4 Videos
- CARBOXYLIC ACIDS AND THEIR DERIVATIVESBook:OP TANDONChapter:CARBOXYLIC ACIDS AND THEIR DERIVATIVESExercise:Brain storming problems
Explore 30 Videos
- BASIC PRINCIPLES OF ORGANIC COMPOUNDS (MECHANISM OF ORGANIC REACTIONS)Book:OP TANDONChapter:BASIC PRINCIPLES OF ORGANIC COMPOUNDS (MECHANISM OF ORGANIC REACTIONS)Exercise:SINGLE INTEGER ANSWER TYPE QUESTIONS
Explore 4 Videos
- CHEMICAL KINETICSBook:OP TANDONChapter:CHEMICAL KINETICSExercise:LINKED COMPRESHENSION TYPE QUESTIONS ( SECTION-VI)
Explore 4 Videos
Similar Questions
Give the main products of the reactions :
(a) C6H6HNO3−−−→H2SO4
(b) C6H5CH3CrO2Cl2−−−−→
(c) C6H5CH2CH2CH3Alk.KMNO4−−−−−−−→
(d) C6H6+Cl2sunlight−−−−→
(e) C6H6+CH3COOClAnhyd.AlCl3−−−−−−−→
View SolutionPredict the major products in the following reaction:
(i). C6H6+(CH3)2CHCH2OHH2SO4−−−→ . . .
(ii). C6H5C2H51.Br2 heat,light−−−−−−−−−→2.NaCN . . .
View Solution
Knowledge Check
The intermediate(s) formed during the reaction
C6H5CH2COOAg+Br2CCl4−−→C6H5CH2Br is (are) :AC6H5−CH2−O∣∣OBrBC6H5−CH2−O∣∣∗OCC6H5−.CH2D.BrThe intemediates formed during the reaction:
C6H5CH2COOAg+Br2CCl4−−→ΔC6H5CH2BrAC6H5−CH2−O||C−O.BC6H5.CH2CBr.D
The intermediates formed during the reaction :
C6H5CH2COOAg+Br2CCl4−−→ΔC6H5CH2BrA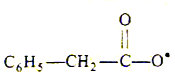 BC6H5.CH2CBr∗D
BC6H5.CH2CBr∗D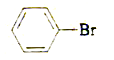
Why CH3−O−CH2−Cl reacts with iodlde ion acetone thousand times faster than CH3Cl?
(ii) Suggest a mechanism for the following reaction.
C6H5O−CH2−CH2−Br(i)Mg/Ether−−−−−−−−→(ii)H2OC6H5OH+CH2=CH2+Mg(OH)Br
View SolutionThe reaction, C6H5CONH2Br2+KOH−−−−−−→ΔC6H5NH2 is called
View SolutionThe reaction,
C6H5Br+2Na+BrCH3→C6H5.CH3+2NaBr is known as
View SolutionDuring the conversion :
C6H5CH2CH3(a)−−→X(b)−−→C6H5CH=CH2
the reagents (a) and (b) are respectively
View SolutionThe major product of the following reaction is:
C6H5CH2−CH3∣C∣Br−CH2−CH3C2H5Na−−−−→C2H5OH
View Solution

