In the given reaction maximum yield is obtained when (X) is:
C6H5COOC2H5+(X)(i)C2H5Ona−−−−−−−→(ii)H+β−ketoester
CH3−CH∣CH3−COOC2H5
CH3−CH3o∣CH3verdet(∣)C−COOC2H5
C6H5−CH∣CH3−COOC2H5
CH3COOC2H5
The correct Answer is:D
Topper's Solved these Questions
- CARBOXYLIC ACIDS AND THEIR DERIVATIVESBook:OP TANDONChapter:CARBOXYLIC ACIDS AND THEIR DERIVATIVESExercise:Integer
Explore 4 Videos
- CARBOXYLIC ACIDS AND THEIR DERIVATIVESBook:OP TANDONChapter:CARBOXYLIC ACIDS AND THEIR DERIVATIVESExercise:Brain storming problems
Explore 30 Videos
- BASIC PRINCIPLES OF ORGANIC COMPOUNDS (MECHANISM OF ORGANIC REACTIONS)Book:OP TANDONChapter:BASIC PRINCIPLES OF ORGANIC COMPOUNDS (MECHANISM OF ORGANIC REACTIONS)Exercise:SINGLE INTEGER ANSWER TYPE QUESTIONS
Explore 4 Videos
- CHEMICAL KINETICSBook:OP TANDONChapter:CHEMICAL KINETICSExercise:LINKED COMPRESHENSION TYPE QUESTIONS ( SECTION-VI)
Explore 4 Videos
Similar Questions
In the given reaction : C2H5O−O∣∣C−(CH2)3−CH2−COOC2H5(i)C2H5ONa/C2H5OH−−−−−−−−−−−−−−→(ii)H2O/HCl[X]
[X] is :
View SolutionGiven the products in each of the following reactions :
(i) H3C−CH2−O∣∣C−CH3C6H5−CO3H−−−−−−−−→,C2H5Ona−−−−−→H+
(ii) H3C−CHOC6H5−CO3H−−−−−−−−→CH3−CH2ONa−−−−−−−−−→H+
(iii) H3C−C≡CHHgSO4/H2SO4−−−−−−−−−→,C6H5−CO3H−−−−−−−−→CH3ONa−−−−−→H+
View Solution
Knowledge Check
The organic product formed in the reaction
C6H5COOCH3(i)LiAlH4−−−−−−−−→(ii)H2OAC6H5CH2OH and CH3OHBC6H5COOH and CH4CC6H5CH3andCH3 OHDC6H5CH3andCH4 (i)Br2,H+−−−−−−−−→(ii)C6H5OnaP,P will beA
(i)Br2,H+−−−−−−−−→(ii)C6H5OnaP,P will beA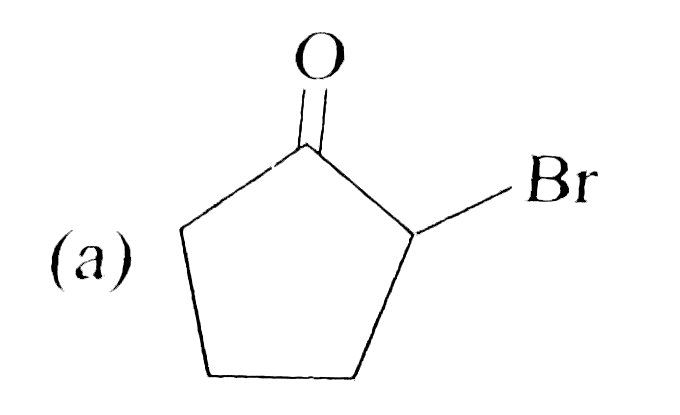 B
B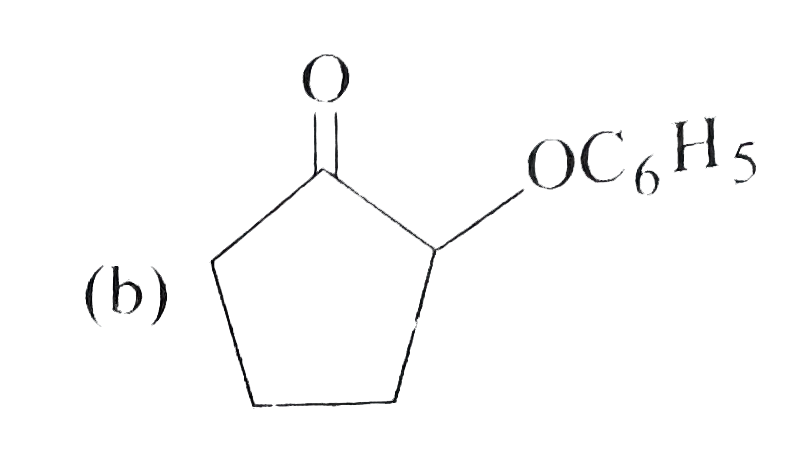 C
C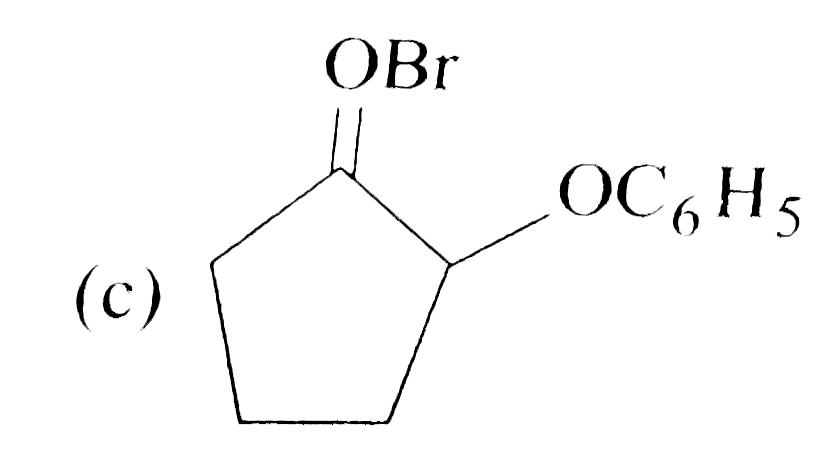 D
D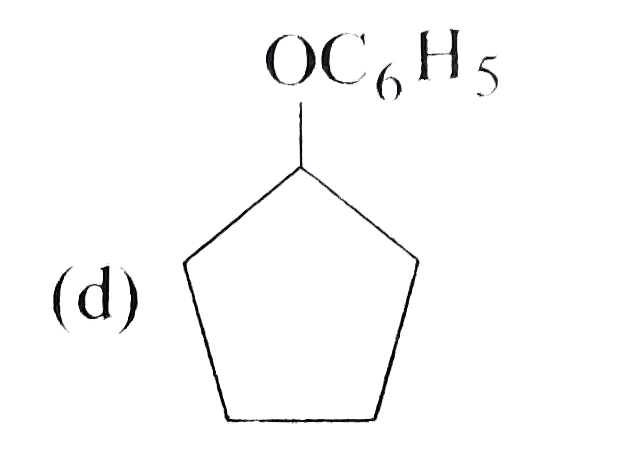
Ester given nucleophilic addition reaction followed by elimination reaction with carbon nucleophile . When carbon nucleophile is of an ester then the reaction is know as Claisen condensation reaction. This reactions is also carrie out between ester and a ketone. A successful Claisen condensation requires an ester with two α - hydrogens and a an equivalent amount of base rather than a cataytic amount of base .
In the give reaction .
C2H5O−O∣∣C−(CH2)5−CH2−COOC2H5(i)C2H3ONa/C2H5OH−−−−−−−−−−−−−−→(ii)H2O/HCl[X] [X] is .A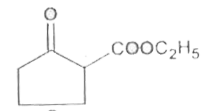 B
B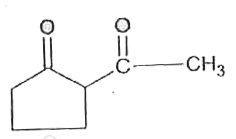 C
C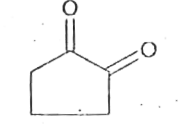 D
D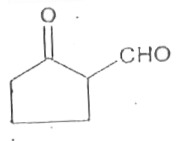
CH3COOH+C2H5OHX−−→CH3COOC2H5+H2O,X is
View SolutionIn the following Cross-Clasisen condensation reaction, how many different β−ke→ esters would be formed?
CH3CH2COOC2H5+CH3COOC2H5C2H5ONa−−−−−→heat
View SolutionIn the given reaction
C6H5−O∣∣C−CH3(i). Br2/KOH−−−−−−−−−−→(ii). H⊕CHBr3+[X]
[X] will be:
View SolutionThe reaction CH3COOC2H5+H2O+H2OH+−−→CH3COOH+C2H5OH is of
View SolutionIn the given reaction
C6H5CHO+X(i)Zn−−−−−−−−−−−→(ii)HOH/NH4ClC6H5−OH∣∣CH−CH2−COOC2H5,[X] will be
View Solution

