(A) Ethyl acetate is more reactive than acetamide towards nucleophilic substitution.
(R) −OC2H5 is more electron attracting than −NH2 group.
A
If both (A) and ( R) are correct and ( R) is the correct explanation of (A).
B
If both (A) and (R) are correct but (R) is not correct explanation of (A).
C
If (A) is correct but ( R) is incorrect.
D
If (A) is incorrect but ( R) is correct.
Video Solution
Text Solution
The correct Answer is:A
|
Answer
Step by step video, text & image solution for (A) Ethyl acetate is more reactive than acetamide towards nucleophilic substitution. (R) -OC_(2)H_(5) is more electron attracting than - NH_(2) group. by Chemistry experts to help you in doubts & scoring excellent marks in Class 12 exams.
Updated on:21/07/2023Similar Questions
Knowledge Check
Aryl halide is less reactive than halide towards nucleophilic substitution because
ALess stable carbonium ionBDue to large C-Cl bond energyCInductive effectDResonance stabilization and sp2 hybridisation of C attached to halideAryl halide is less reactive than alkyl halide towards nucleophilic substitution because
ALess stable carbonum ionBDue to large C-Cl bond energyCInductive effectDResonance stabilization and sp2-hybridisation of C attached to halideWhich is least reactive towards nucleophilic substitution (SN2)
ACH3=CH−CH2ClB CH3 |CH3−C−Cl | CH3C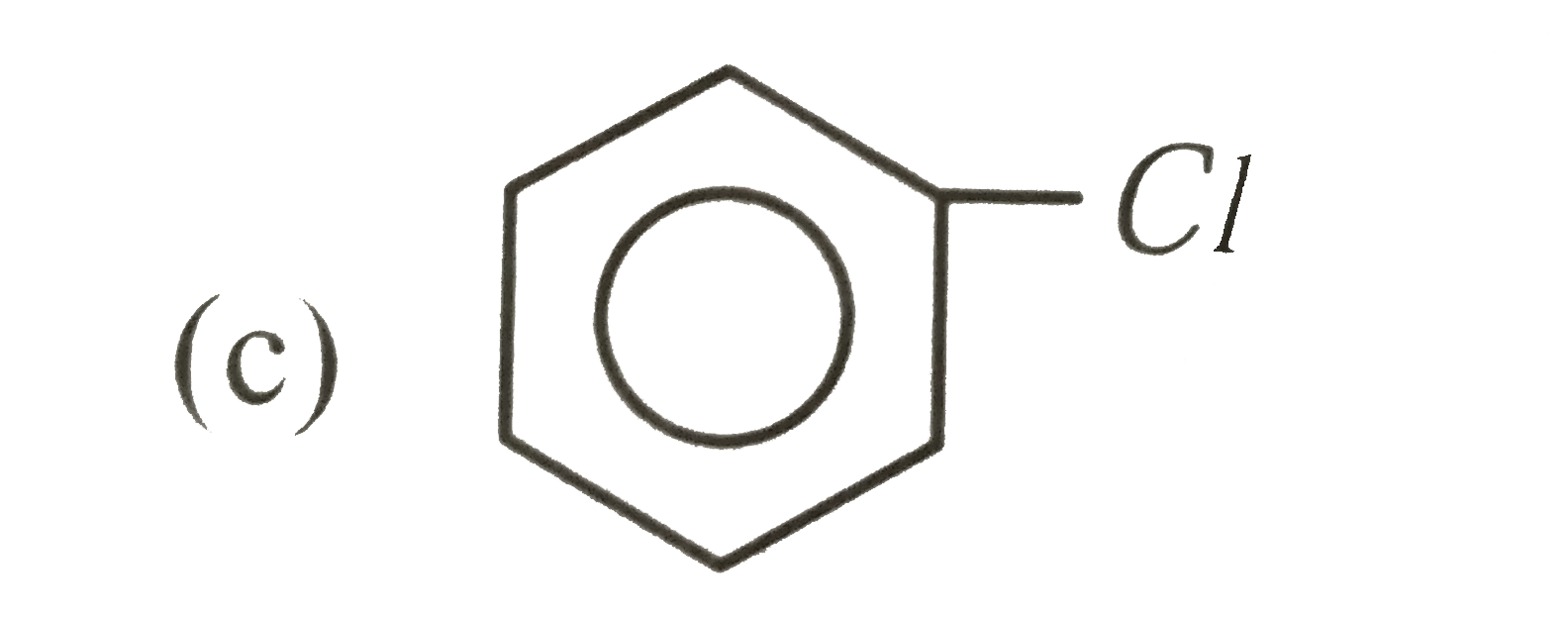 DCH3−CH(Cl)CH3
DCH3−CH(Cl)CH3

