For a gaseous system, change in internal energy and work done on the system are respectively 17 J and 41 J. find heat supplied / evolved from the system.
ΔQ=17−41=−24J, evolved by 1st law.
Topper's Solved these Questions
- THERMODYNAMICSBOOK - AAKASH INSTITUTECHAPTER - THERMODYNAMICSEXERCISE - EXERCISE
19 Videos
- THERMODYNAMICSBOOK - AAKASH INSTITUTECHAPTER - THERMODYNAMICSEXERCISE - ASSIGNMENT (SECTION -A) (Objective Type Questions)
39 Videos
- THERMODYNAMICSBOOK - AAKASH INSTITUTECHAPTER - THERMODYNAMICSEXERCISE - EXAMPLE
44 Videos
- THERMAL PROPERTIES OF MATTERBOOK - AAKASH INSTITUTECHAPTER - THERMAL PROPERTIES OF MATTEREXERCISE - Assignment (Section-J) Akash Challengers Questions
7 Videos
- UNITS AND MEASUREMENTSBOOK - AAKASH INSTITUTECHAPTER - UNITS AND MEASUREMENTSEXERCISE - ASSIGNMENT (SECTION - D)
15 Videos
Similar Questions
Two bodies A and B are said to be in thermal equilibrium with each other if they have same
View SolutionState Zeroth law of thermodynamics. How does it lead to the concept of temperature?
View SolutionWhen are the two bodies in thermal equilibrium?
View SolutionA slab of cunductivity k is in the shape of trapezoid as shown I figure. If the two end faces be maintained at temperature T1=50∘CandT2=25∘C, then determine the thermal current through the slab by ignoring any heat loss through the lateral surfaces.
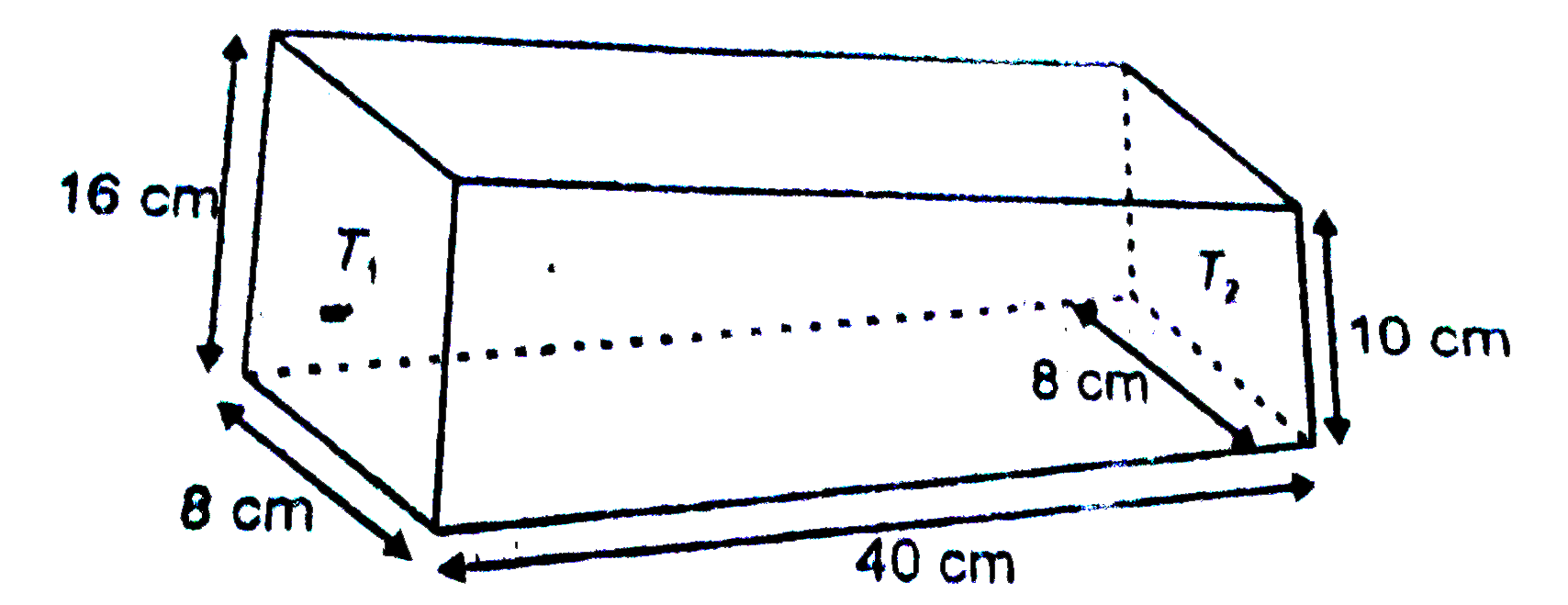 .
.
View SolutionA : PARSEC and light year, both measure time
R : Both have dimension of time.
View SolutionFor a gaseous system find change in internal energy if the heat supplied to the system is 50 J and work done by the system is 16 J.
View SolutionFor a gaseous system, change in internal energy and work done on the system are respectively 17 J and 41 J. find heat supplied / evolved from the system.
View SolutionFind the change in internal energy if heat supplied to the gaseous system is 100 J and work done by the system is 80 J.
View SolutionCalculate the changes in internal energy of a system if,
300 J of heat energy is added to the system and a work of 160 J is done on the system.
View SolutionFor a gaseous system find change in internal energy if the heat supplied to the system is 50 J and work done by the system is 16 J.
View SolutionWhen 110J of heat is supplied to a gaseous system, the internal energy of the system increase by 40J. The amount of external work done (in J) is
View SolutionFor a gaseous state if heat supplied to the system is 100 J and work done by the system is 25 J. then internal energy of the system is
View SolutionThe work done by a system is 8 J, when 40 J heat is supplied to it. The change in internal energy of the system during the process:
View SolutionFor a gaseous system, if heat rejected by the system is 100 J and work done by the system is 20 J , then change in internal energy is
View Solution______is the change in internal energy of a system if on giving 40 J of heat of the system, the work done on the system is 8 J.
View Solution

