Dihydrogen gas used in Haber's process is produced by reacting methane from natural gas with high temperature steam. The first stage of the two 2 stage reaction involves the formation of CO and H2. In second stage, CO formed in first stage is reacted with more steam in water gas shift reaction,
CO(g)+H2O(g)⇔CO2(g)+H2(g)
If a reaction vessel at 400∘C is charged with an equimolar mixture of CO and steam such that pCO=pH2O=4.0 bar, what will be the partial pressure of H2 at equilibrium? Kp=0.1 at 400∘C.
Given: PCO=PH2O=4.0 Bar, KP=10.1
Asked: Partial pressure of H2 at equilibrium
Formulae: KP=(PCO2)(PH2)(PCO)(PH2O)
Substitution & Calculation:
Suppose the partial pressure of H2 at equilbrium=P¯¯¯¯¯¯<br>CO(g)+H2O(g)⇔CO2(g)+H2(g)
Initial pressure 4.0bar4.0 bar
At eqm. (4−P)(4−P)PP
KP=P2(4−P)2=0.1 (Given)
∴P4−P=√0.1=0.316
P=1.264−0.316Por1.316P=1.264orP=0.96 bar
Topper's Solved these Questions
- CHEMICAL EQUILIBRIUMBOOK - RESONANCECHAPTER - CHEMICAL EQUILIBRIUMEXERCISE - Exercise-1 (Part-1)
38 Videos
- CHEMICAL EQUILIBRIUMBOOK - RESONANCECHAPTER - CHEMICAL EQUILIBRIUMEXERCISE - Exercise-1 (Part-2)
88 Videos
- CHEMICAL EQUILIBRIUMBOOK - RESONANCECHAPTER - CHEMICAL EQUILIBRIUMEXERCISE - (MSPs)
8 Videos
- CHEMICAL BONDINGBOOK - RESONANCECHAPTER - CHEMICAL BONDINGEXERCISE - Inorganic chemistry (Chemistry Bonding)
49 Videos
- D & F-BLOCK ELEMENTS & THEIR IMPORTANT COMPOUNDS BOOK - RESONANCECHAPTER - D & F-BLOCK ELEMENTS & THEIR IMPORTANT COMPOUNDS EXERCISE - Match the column
1 Video
Similar Questions
In a reaction A+B⇔C+D the rate constant of forward reaction & backward reaction is K1 and K12 then the equilibrium constant (K) for reaction is expressed as:
View SolutionA reversible reaction is one which
View SolutionAn example of a reversible reaction is
View SolutionWhich is correct order ofr net dipole moment :
View SolutionMatch the reactions in Column I with the types of products/the use of products in Column II.
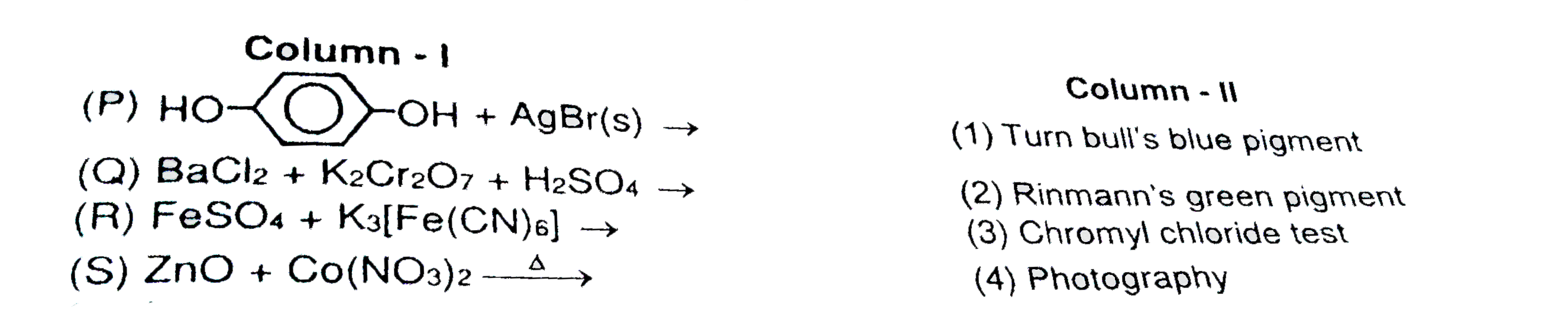
View SolutionFor the water gas reaction: C(s) +H_(2)O(g) hArr CO(g) +H_(2)(g) the standard Gibbs enegry for the reaction at 1000K is -8.1 kJ mol^(-1) . Calculate its equilibriu constant.
View SolutionDihydrogen gas used in Haber's process is produced by reacting methane from natural gas with high temperature steam. The first stage of the two 2 stage reaction involves the formation of CO and H_(2) . In second stage, CO formed in first stage is reacted with more steam in water gas shift reaction, CO(g)+H_(2)O(g) hArr CO_(2)(g)+H_(2)(g) If a reaction vessel at 400^(@)C is charged with an equimolar mixture of CO and steam such that p_(CO)=p_(H_(2)O)=4.0 bar, what will be the partial pressure of H_(2) at equilibrium? K_(p)=10.1 at 400^(@)C .
View SolutionIn the production of dihydrogen gas via water gas shift reaction CO(g)+H_(2)(g) underset("Catalyst")overset("heat")to CO_(2)(g)+H_(2)(g) CO_(2) gas is removed by scrubbing with solution of
View SolutionOne mole of H_(2)O and one mole of CO are taken in a 10 litre vessel and heated at 725 K. At equilibrium 40% of water (by mass) reacts with CO according to the equation : H_(2) O (g) + CO(g) hArr H_(2)(g) + CO_(2) (g) calculate the equilibrium constant for the reaction.
View SolutionDihydrogen gas used in Haber's process is produced by reacting methane from natural gas with high temperature steam. The first stage of the two 2 stage reaction involves the formation of CO and H2. In second stage, CO formed in first stage is reacted with more steam in water gas shift reaction,
CO(g)+H2O(g)⇔CO2(g)+H2(g)
If a reaction vessel at 400∘C is charged with an equimolar mixture of CO and steam such that pCO=pH2O=4.0 bar, what will be the partial pressure of H2 at equilibrium? Kp=0.1 at 400∘C.
View Solutionहाबर विधि में प्रयुक्त हाइड्रोजन को प्राकृतिक गैस से प्राप्त मेथेन को उच्च ताप की भाप से क्रिया करके बनाया जाता हैं। दो पदों वाली अभिक्रिया के प्रथम पद में, प्रथम प् दमे बनने वाली CO और अधिक भाप से क्रिया करती हैं।
CO(g)+H2O(g)<⇒CO2(g)+H2(g)
यदि 400∘ C अभिक्रिया पात्र में CO एवं भाप का सम-मोलर मिश्रण इस प्रकार लिया जाये की PCO=PH2O=4.0 bar तो H2 का साम्यावस्था पर आंशिक दाब क्या होगा? (400∘C) पर Kp=0.1
View SolutionAt 675 K, H2(g) and CO2(g) react to form CO(g) and H2O(g),Kp for the reaction is 0.16.
If a mixture of 0.25 mole of H2(g) and 0.25 mol of CO2 is heated at 675 K, mole% of CO(g) in equilibrium mixture is :
View SolutionOne mole of H_(2)O and one mole of CO are taken in 10 L vessel and heated to 725 K. at equilibrium 40% of water (by mass) reacts with CO according to the equation H_(2)O(g)+CO(g)hArr H_(2)(g)+CO_(2)(g) . Calculate the equilibrium constant for the reaction.
View SolutionDihydrogen gas used in Haber's process is produced by reacting methane from natural gas with high temperature steam. The first stage of two stage reaction involves the formation of CO and H_(2) . In second stage, CO formed in first stage is reacted with more steam in water gas shift reaction. CO(g)+H_(2)O(g)hArr CO_(2)(g)+H_(2)(g) If a reaction vessel at 400^(@)C is charged with an equimolar mixture of CO and steam such that p_(CO)=p_(H_(2)O)=4.0 bar, what will be the partial pressure of H_(2) at equilibrium? K_(p)=10.1 at 400^(@)C .
View SolutionThe reaction was started with 0.1 M each of CO and H_(2)O at 800K. K_(C) for the reaction, CO(g)+H_(2)O (g) Leftrightarrow CO_(2) (g)+H_(2) (g) at 800K, 4.24. What is the equilibrium concentration of CO_(2) gas?
View SolutionDihydrogen gas used in Haber's process is produced by reacting methane from natural gas with high temperature steam. The first stage of two stage reaction involves the formation of CO and H_(2) . In second stage , CO formed in first stage is reacted with more steam in water gas shift reaction. CO(g)+H_(2)OhArrCO_(2)(g)+H_(2)(g) If a reaction vessel at 400^(@)C is charged with an equimolar mixtureof CO and steam such that p_(CO)=p_(H_(2)P=4.0 bar, what will be the partial pressure of H_(2) at equilibrium ? K_(p)=10.1 at 400^(@)C
View Solution

