Attainment of the equilibrium A(g)⇔2C(g)+B(g)gave the following graph . Find the correct option .(%dissociation=Frationdissoiatedxx100)
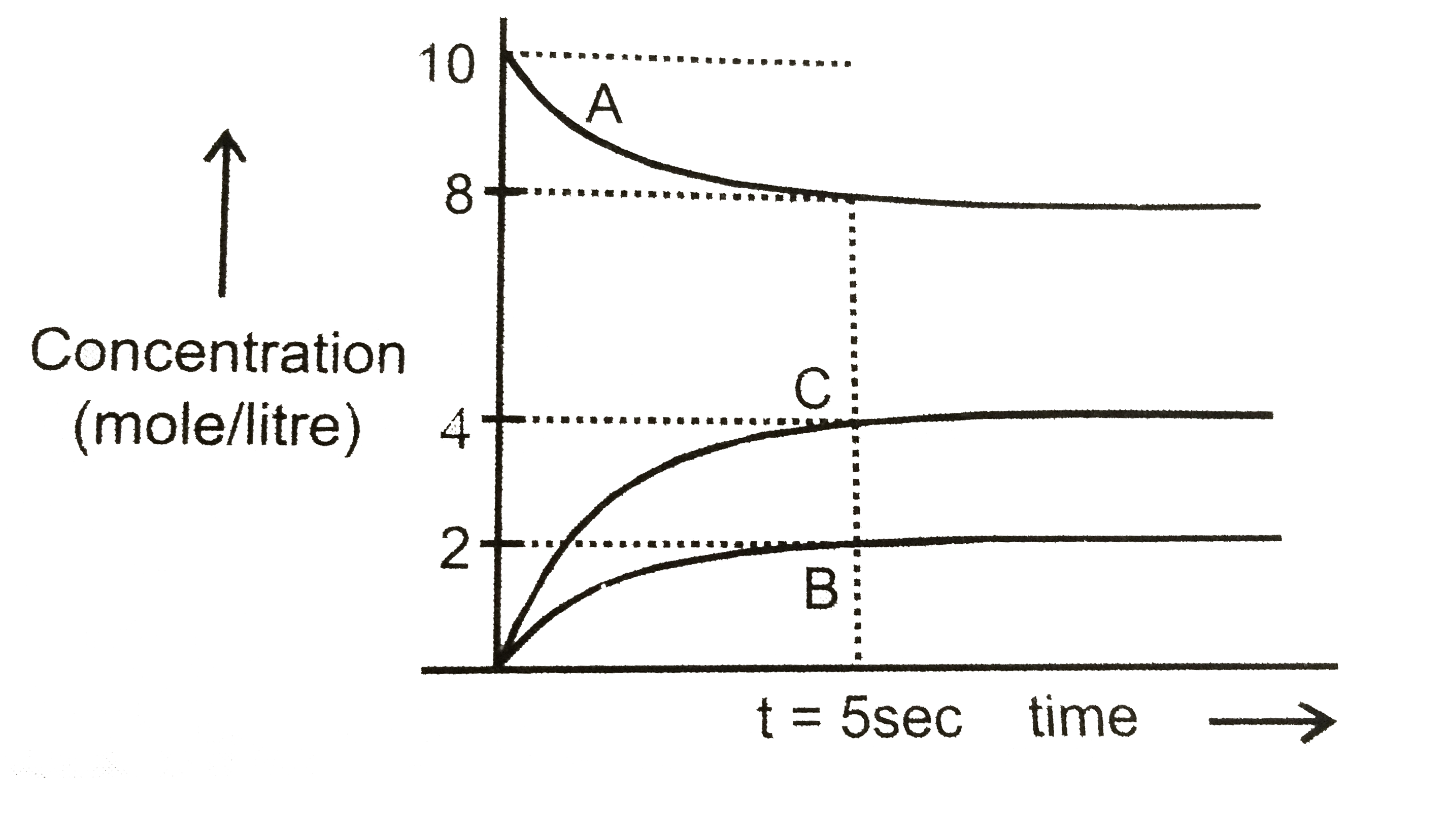
At =5sec equilibrium has been reached andKP=128(mol//litre)2
At t=5sec equilibrium has been reached and%dissociation ofAis60%
At t=5sec equilibrium has been reached and%dissociation ofAis40%
None of these
The correct Answer is:C
Step by step video, text & image solution for Attainment of the equilibrium A(g)hArr2C(g)+B(g)gave the following graph . Find the correct option .(% dissociation=Fration dissoiatedxx100) by Chemistry experts to help you in doubts & scoring excellent marks in Class 11 exams.
Updated on:21/07/2023Topper's Solved these Questions
Similar Questions
Knowledge Check
Attainment of the equilibrium A(g)⇔2C(g)+B(g)gave the following graph . Find the correct option .(% dissociation=Fration dissoiated×100)
 AAt t=5 sec equilibrium has been reached and Kc=40(mol/litre)2BAt t=5 sec equilibrium has been reached and % dissciation of A is 20%CAt t=5 sec equilibrum has been reached and % dissocition of A is 30%Dnone of these
AAt t=5 sec equilibrium has been reached and Kc=40(mol/litre)2BAt t=5 sec equilibrium has been reached and % dissciation of A is 20%CAt t=5 sec equilibrum has been reached and % dissocition of A is 30%Dnone of theseConsider the following equilibrium A(g)⇔2B(g) and the following graph : Select the correct statements.
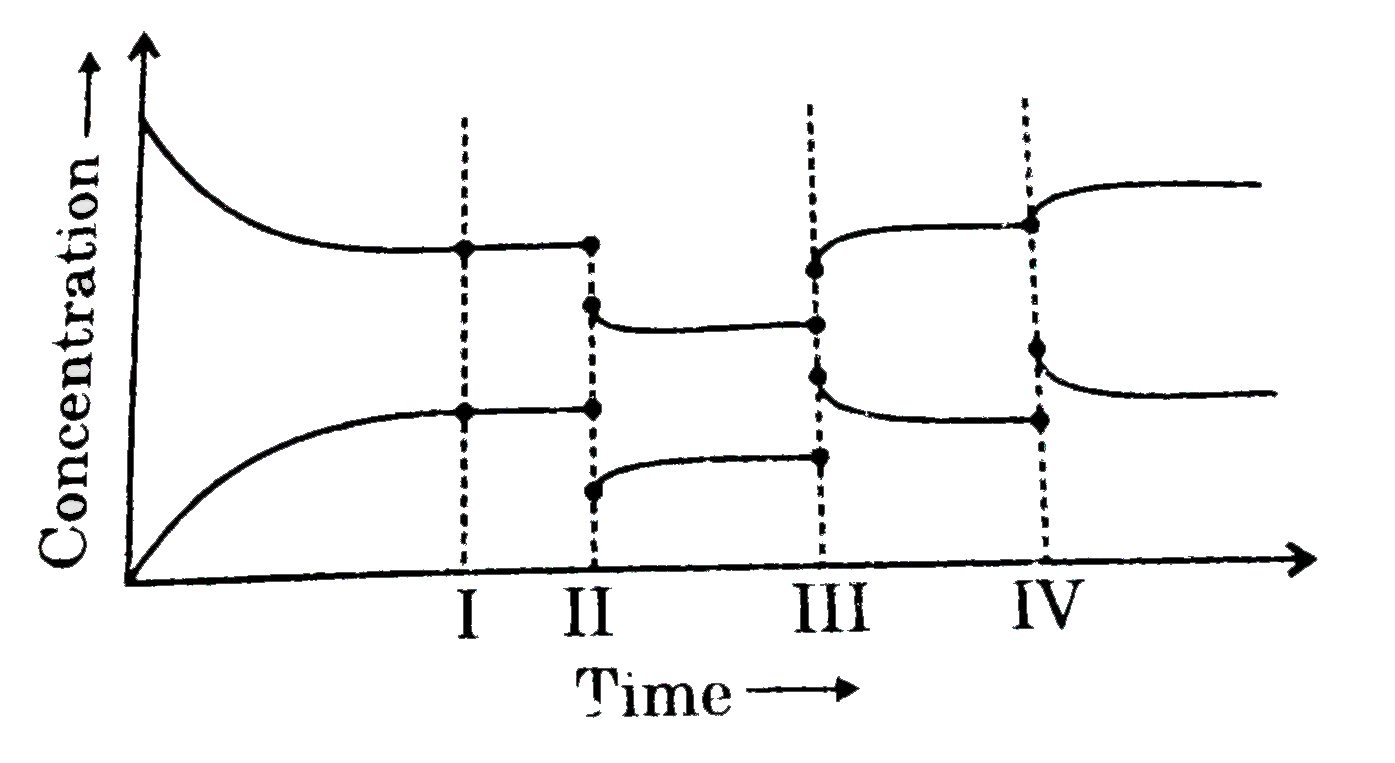 AEffect (I) represent the insertion of inert gas at constant volumeBEffect (II) represent the insertion of inert gas at constant pressureCEffect (III) represent the decrease in volume of containerDEffect (IV) represent the addition of moles of gas B
AEffect (I) represent the insertion of inert gas at constant volumeBEffect (II) represent the insertion of inert gas at constant pressureCEffect (III) represent the decrease in volume of containerDEffect (IV) represent the addition of moles of gas BFor the equilibrium
2NO(g)+Cl2(g)⇔2NOCl(g)
Kp is related to Kc by the reactionAKp=Kc(RT)BKp=Kc(RT)2CKp=Kc/RTDKp=Kc/(RT)2
For the equilibrium AB(g)⇔A(g)+B(g) at a given temperature, the pressure at which one-third of AB is dissociated is numerically equal to
View SolutionFor the equilibrium
2NO(g)+Cl2(g)↔2NOCl(g)
Kp is related to Kc by the reaction:
View SolutionConsider the imaginary equilibrium 4A(g)+5B(g)⇔4X(g)+6Y(g), The unit of equilibrium constant KC is
View SolutionFor the chemical reaction
N2(g)+3H2(g)⇔2NH3(g)
The correct option is:
View SolutionFor the chemical reaction
N2(g)+3H2(g)⇔2NH3(g)
The correct option is:
View Solution