A2 lit vessel is filled by 1 mole of each gas A&B. If KC for reaction A(g)⇔B(g) is 1.5 at temp. T. [Atomic mass of A is 40 & Bis20]. 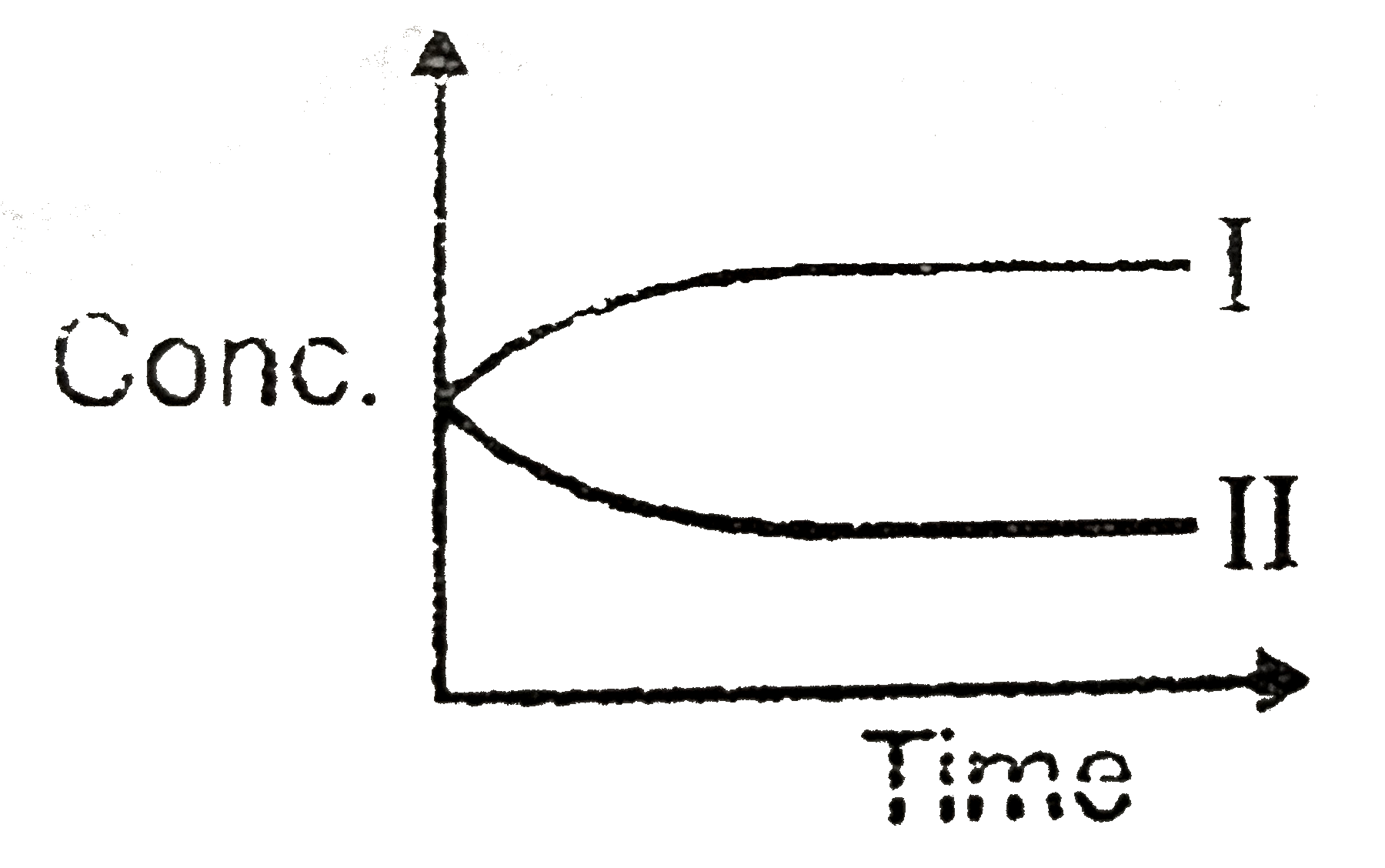
[A] vs time is graph I
[B] vs time is graph I
[A] vs time is graph II
[B] vs time is grap II
The correct Answer is:B, C
Topper's Solved these Questions
Similar Questions
Which is correct order ofr net dipole moment :
View SolutionFor the reaction A(g)+3B(g)⇔2C(g) at 27∘C.2 mole of A, 4 moles of B and 6 moles of C are present in 2 lit vessel. If Kc for the reaction is 1.2, the reaction will proceed in
View SolutionA के 3 मोल और B के 1 मोल को जब 1 लीटर के बर्तन में अभिकृत करवाया जाता है तो Ag+Bg→2Cg अभिक्रिया होती है तथा C के 1.5 मोल बनते हैं, अभिक्रिया के लिए साम्य स्थिरांक होगा
View SolutionFor the reaction A(g)+3B(g)⇔2C(g)at 27∘C, 2 moles of A, 4 moles of B and 6 moles of C are present in 2 litre vessel. If Kc for the reaction is 1.2, the reaction will proceed in :
View Solution5 litre vessel contains 2 moles of each of gases A and B at equilibrium. If 1 mole each of A and B are removed. Calculate KC for the reaction A(g)⇔B(g)
View SolutionFor the reaction A(g)+3B(g)⇔2C(g)at 27∘C, 2 moles of A, 4 moles of B and 6 moles of C are present in 2 litre vessel. If Kc for the reaction is 1.2, the reaction will proceed in :
View Solution1 mole of a gas A is taken in a vessel of volume 1L . It dissociates according to the reaction A(g)hArrB(g)+C(g) "at" 27^(@)C . Forward and backward reaction rate constants for the reaction are 1.5xx10^(-2) "and" 3xx10^(-2) respectively. Find the concentrations of A,B "and" C at equilibrium.
View SolutionA2 lit vessel is filled by 1 mole of each gas A&B. If KC for reaction A(g)⇔B(g) is 1.5 at temp. T. [Atomic mass of A is 40 & Bis20].

View Solution

