If reaction A+B⇔C+D, taken place in 5 liter close vessel, the rate constant of forward reaction is nine times of rate of backward reaction.
If initially one mole of each reactant present in the container, then find the correct option//is.
[C][B]=31
logKP=logKC
[D]eq=15×10−2moleL−1
Keq=9
The correct Answer is:A, B, C, D
A+B⇐⇒C+D1−x51−x5x5x5t=eq
KF=9KbKC=KFKb=91
KC=(5/5)2(1−x5)2=91x1−x=3
x=3−3x
4x=3
x=34=0.75
[A]=0.255×100=5×10−2moleL−1[B]=[A]C=0.75500=15×10−2molL−1
Topper's Solved these Questions
Similar Questions
If the rate constant of forward reaction is triple of rate constant of backward reaction under identical condition at equilibrium, then Keq for the reaction is
View SolutionIf the rate constant of forward reaction is triple rate constant of backward reaction under identical condition at equilibrium then Keq for the reaction is
View Solution
Knowledge Check
The rate constant for a first order reaction is equal to the initial rate of reaction when the initial concentration of the reactant is
A100MB1×10−2 MC1.0 MD0.1 MThe equilibrium constant of the reaction A+B⇔C+D is 10. If the rate constant of forward reaction is 203, the rate constant for backward reaction is
A20.3B10.3C2.03D203At equilibrium for a reaction A↔B.
correct representation is {rf= rate of forward reaction, rb = rate of backward reaction}A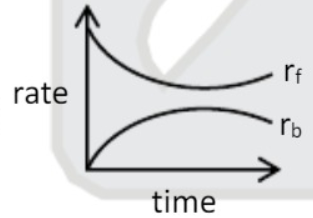 B
B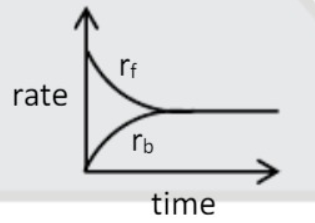 C
C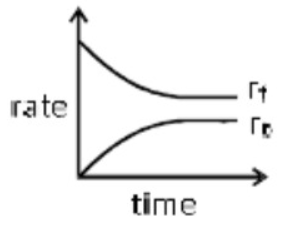 D
D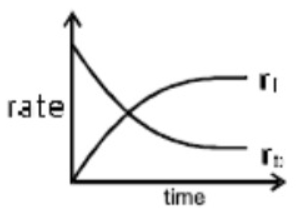
In the reaction A+B⇔C+D, the value of equilibrium constant is 10. If the rate constant of forward reaction is 80, the rate constant of backward reaction is …………
View SolutionIn the reaction A+B⇔C+D, the value of equilibrium constant is 10. If the rate constant of forward reaction is 80, the rate constant of backward reaction is …………
View SolutionIn the reaction A+B⇔C+D , the value of equilibrium constant is 10 . If rate constant of forward reaction is 105 , the rate constant of backward reaction is …………
View SolutionIn the reaction A+B⇔C+D , the value of equilibrium constant is 10 . If rate constant of forward reaction is 105 , the rate constant of backward reaction is …………
View SolutionAt equilibrium for a reaction A↔B.
correct representation is {rf= rate of forward reaction, rb = rate of backward reaction}
View Solution

