Identify a molecule which does not exist.
He2
Li2
C2
O2
The correct Answer is:A
To identify a molecule that does not exist, we will calculate the bond order for each of the given options. The bond order is determined by the formula:
Bond Order=12(Number of bonding electrons−Number of antibonding electrons)
A bond order of zero indicates that the molecule does not exist.
1. Analyze the first option: Helium (He₂)
- Helium has an atomic number of 2, which means it has 2 electrons.
- For He₂, the total number of electrons = 2 (from one He) + 2 (from another He) = 4 electrons.
- The electronic configuration is:
\sigma_{1s}^2 \sigma^*_{1s}^2
- Bonding electrons = 2 (from σ1s), Antibonding electrons = 2 (from σ∗1s).
- Calculate the bond order:
Bond Order=12(2−2)=12(0)=0
- Since the bond order is 0, He₂ does not exist.
2. Analyze the second option: Lithium (Li₂)
- Lithium has an atomic number of 3, which means it has 3 electrons.
- For Li₂, the total number of electrons = 3 (from one Li) + 3 (from another Li) = 6 electrons.
- The electronic configuration is:
\sigma_{1s}^2 \sigma^*_{1s}^2 \sigma_{2s}^2
- Bonding electrons = 4 (2 from σ1s and 2 from σ2s), Antibonding electrons = 2 (from σ∗1s).
- Calculate the bond order:
Bond Order=12(4−2)=12(2)=1
- Since the bond order is 1, Li₂ exists.
3. Analyze the third option: Dicarbon (C₂)
- Carbon has an atomic number of 6, which means it has 6 electrons.
- For C₂, the total number of electrons = 6 (from one C) + 6 (from another C) = 12 electrons.
- The electronic configuration is:
\sigma_{1s}^2 \sigma^*_{1s}^2 \sigma_{2s}^2 \sigma^*_{2s}^2 \pi_{2p_x}^2 \pi_{2p_y}^2
- Bonding electrons = 8 (2 from σ1s, 2 from σ2s, 2 from π2px, 2 from π2py), Antibonding electrons = 4 (2 from σ∗1s and 2 from σ∗2s).
- Calculate the bond order:
Bond Order=12(8−4)=12(4)=2
- Since the bond order is 2, C₂ exists.
4. Analyze the fourth option: Dioxygen (O₂)
- Oxygen has an atomic number of 8, which means it has 8 electrons.
- For O₂, the total number of electrons = 8 (from one O) + 8 (from another O) = 16 electrons.
- The electronic configuration is:
\sigma_{1s}^2 \sigma^*_{1s}^2 \sigma_{2s}^2 \sigma^*_{2s}^2 \sigma_{2p_z}^2 \pi_{2p_x}^2 \pi_{2p_y}^2 \pi^*_{2p_x}^1 \pi^*_{2p_y}^1
- Bonding electrons = 10, Antibonding electrons = 6.
- Calculate the bond order:
Bond Order=12(10−6)=12(4)=2
- Since the bond order is 2, O₂ exists.
Conclusion:
The molecule that does not exist is He₂ (option A).
- Helium has an atomic number of 2, which means it has 2 electrons.
- For He₂, the total number of electrons = 2 (from one He) + 2 (from another He) = 4 electrons.
- The electronic configuration is:
\sigma_{1s}^2 \sigma^*_{1s}^2
- Bonding electrons = 2 (from σ1s), Antibonding electrons = 2 (from σ∗1s).
- Calculate the bond order:
Bond Order=12(2−2)=12(0)=0
- Since the bond order is 0, He₂ does not exist.
- Lithium has an atomic number of 3, which means it has 3 electrons.
- For Li₂, the total number of electrons = 3 (from one Li) + 3 (from another Li) = 6 electrons.
- The electronic configuration is:
\sigma_{1s}^2 \sigma^*_{1s}^2 \sigma_{2s}^2
- Bonding electrons = 4 (2 from σ1s and 2 from σ2s), Antibonding electrons = 2 (from σ∗1s).
- Calculate the bond order:
Bond Order=12(4−2)=12(2)=1
- Since the bond order is 1, Li₂ exists.
- Carbon has an atomic number of 6, which means it has 6 electrons.
- For C₂, the total number of electrons = 6 (from one C) + 6 (from another C) = 12 electrons.
- The electronic configuration is:
\sigma_{1s}^2 \sigma^*_{1s}^2 \sigma_{2s}^2 \sigma^*_{2s}^2 \pi_{2p_x}^2 \pi_{2p_y}^2
- Bonding electrons = 8 (2 from σ1s, 2 from σ2s, 2 from π2px, 2 from π2py), Antibonding electrons = 4 (2 from σ∗1s and 2 from σ∗2s).
- Calculate the bond order:
Bond Order=12(8−4)=12(4)=2
- Since the bond order is 2, C₂ exists.
- Oxygen has an atomic number of 8, which means it has 8 electrons.
- For O₂, the total number of electrons = 8 (from one O) + 8 (from another O) = 16 electrons.
- The electronic configuration is:
\sigma_{1s}^2 \sigma^*_{1s}^2 \sigma_{2s}^2 \sigma^*_{2s}^2 \sigma_{2p_z}^2 \pi_{2p_x}^2 \pi_{2p_y}^2 \pi^*_{2p_x}^1 \pi^*_{2p_y}^1
- Bonding electrons = 10, Antibonding electrons = 6.
- Calculate the bond order:
Bond Order=12(10−6)=12(4)=2
- Since the bond order is 2, O₂ exists.
The molecule that does not exist is He₂ (option A).
Topper's Solved these Questions
Similar Questions
Knowledge Check
Which does not exists
A[CF6]2−B[SiF6]2C[GeF6]2D[SnF6]2Molecule which does not oxidise
A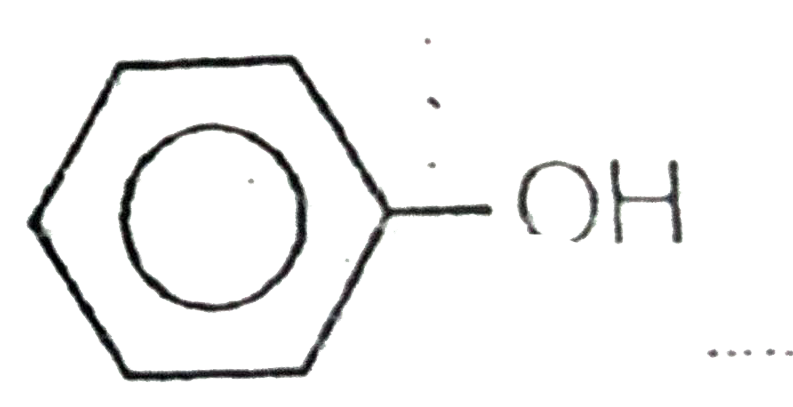 B
B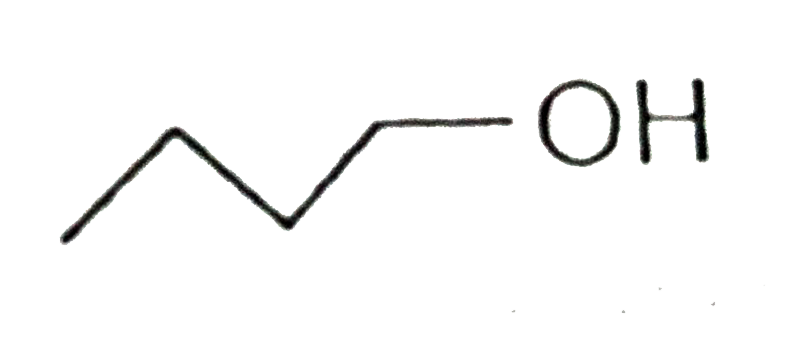 C
C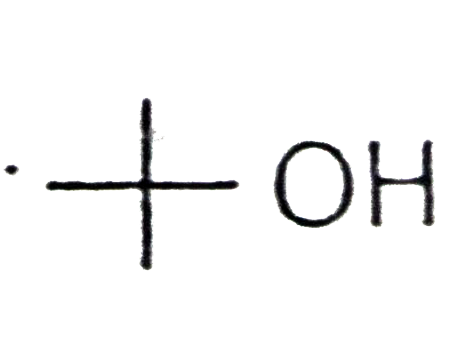 D
D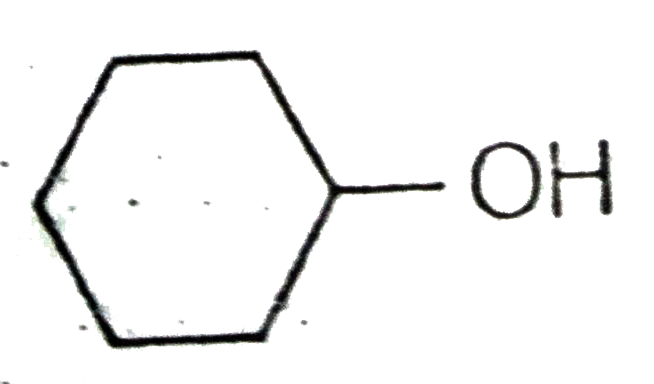
The atomic numbers of the elements X, Y and Z are 2, 10 and 16, respectively. Identify the element which does not exist in monoatomic state.
AXBZCYDX and Y

