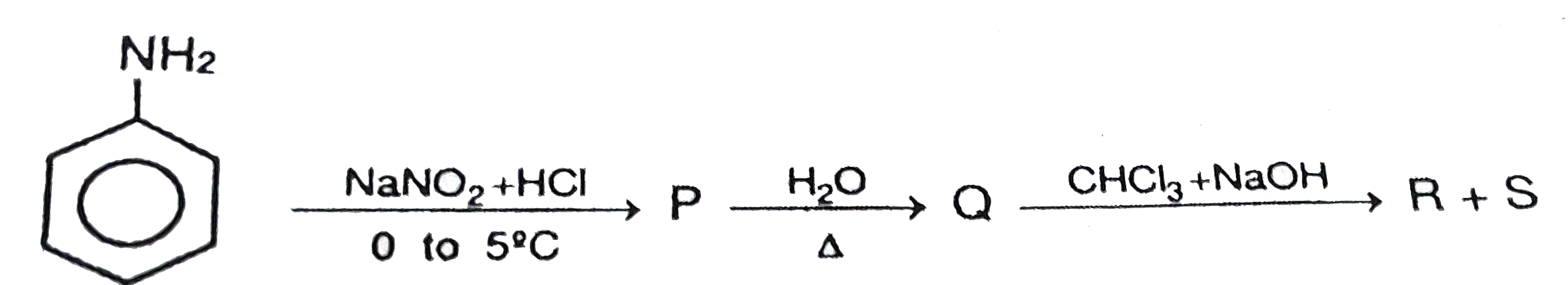
Which of the following statement is/are correct:
A
P is benzene diazonioum chloride
B
Q can not give bromine water test
C
R and S are position isomers
D
During the formation of R and S from Q, formation of a new C-C bond will takes place.
Text Solution
The correct Answer is:A, C, D
|
Topper's Solved these Questions
- AROMATIC COMPOUNDS Book:RESONANCEChapter:AROMATIC COMPOUNDS Exercise:PART IV- COMPREHENSION
Explore 14 Videos
- AROMATIC COMPOUNDS Book:RESONANCEChapter:AROMATIC COMPOUNDS Exercise:Exercise -3 Part-I
Explore 36 Videos
- AROMATIC COMPOUNDS Book:RESONANCEChapter:AROMATIC COMPOUNDS Exercise:PART-II SINGLE OR DOUBLE INTEGER TYPE
Explore 7 Videos
- ATOMIC STRUCTUREBook:RESONANCEChapter:ATOMIC STRUCTUREExercise:PHYSICAL CHEMISTRY(ATOMIC EQUILIBRIUM)
Explore 48 Videos
Similar Questions
Knowledge Check
Which of the following statements is/are correct
AThe reaction.
Bi+4HNO3+3H2O→Bi(NO3)3.5H2O+NO
2.09g of Bi in HNO3 produces 48.5g of bismuth nitrate.
(Atomic weight Bi=209g,MwofBi(NO3)3.5H2O=485g)B4.0g of 63% HNO3 by mass is required to react with 2.09g of BiCThe volume of NO gas produced at STP (1 bar 273K) is 0.227LDThe volume of NO gas prodcued at SATP (1 bar, 298K) is 0.247L.Which of the following statements is/are correct
AAbsolute value of internal energy can't be determinedBAbsolute value of heat content can be determinedCAbsolute value of entropy can be determinedDAll the three E, H and S extensive propertiesWhich of the following statements is/are correct ?
APhysical adsorption is multilayer, non-directional, and non-specific.BOn some cases, solvent may be adsorbed in preference to the solute on the surface of the adsorbent.CChemical adsorption increases with increases in temperature.DDue to adsorption, surface energy increases.
Which of the following statements is/are correct ?
View SolutionWhich of the following statements is/are correct ?
View SolutionWhich of the following statements is/are correct ?
View SolutionWhich of the following statements is/are correct .
View SolutionWhich of the following statements is/are correct ?
View Solution

